Constructive Neuroengineering of Crossing Multi-Neurite Wiring Using Modifiable Agarose Gel Platforms
- PMID: 40558718
- PMCID: PMC12192150
- DOI: 10.3390/gels11060419
Constructive Neuroengineering of Crossing Multi-Neurite Wiring Using Modifiable Agarose Gel Platforms
Abstract
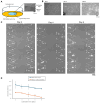
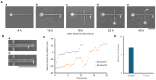
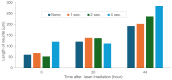
Constructing stable and flexible neuronal networks with multi-neurite wiring is essential for the in vitro modeling of brain function, connectivity, and neuroplasticity. However, most existing neuroengineering platforms rely on static microfabrication techniques, which limit the ability to dynamically control circuit architecture during cultivation. In this study, we developed a modifiable agarose gel-based platform that enables real-time microstructure fabrication using an infrared (IR) laser system under live-cell conditions. This approach allows for the stepwise construction of directional neurite paths, including sequential microchannel formation, cell chamber fabrication, and controlled neurite-neurite crossings. To support long-term neuronal health and network integrity in agarose microstructures, we incorporated direct glial co-culture into the system. A comparative analysis showed that co-culture significantly enhanced neuronal adhesion, neurite outgrowth, and survival over several weeks. The feeder layer configuration provided localized trophic support while maintaining a clear separation between glial and neuronal populations. Dynamic wiring experiments further confirmed the platform's precision and compatibility. Neurites extended through newly fabricated channels and crossed pre-existing neurites without morphological damage, even when laser fabrication occurred after initial outgrowth. Time-lapse imaging showed a temporary growth cone stalling at crossing points, followed by successful elongation in all tested samples. Furthermore, the direct laser irradiation of extending neurites during microstructure modification did not visibly impair neurite elongation, suggesting minimal morphological damage under the applied conditions. However, potential effects on molecular signaling and electrophysiological function remain to be evaluated in future studies. Together, these findings establish a powerful, flexible system for constructive neuroengineering. The platform supports long-term culture, real-time modification, and multidirectional wiring, offering new opportunities for studying neural development, synaptic integration, and regeneration in vitro.
Keywords: agarose gel micropattern; bending angle; bending microchannel array; hippocampal cell; neurite elongation control; neuron; neuronal network formation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Constructive Neuroengineering of Axon Polarization Control Using Modifiable Agarose Gel Platforms for Neuronal Circuit Construction.Gels. 2025 Aug 21;11(8):668. doi: 10.3390/gels11080668. Gels. 2025. PMID: 40868798 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
References
-
- Aebersold M.J., Dermutz H., Forró C., Weydert S., Thompson-Steckel G., Vörös J., Demkó L. “Brains on a chip”: Towards engineered neural networks. Trends Anal. Chem. 2016;78:60–69. doi: 10.1016/j.trac.2016.01.025. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

