Hyaluronic Acid: Production Strategies, Gel-Forming Properties, and Advances in Drug Delivery Systems
- PMID: 40558723
- PMCID: PMC12192020
- DOI: 10.3390/gels11060424
Hyaluronic Acid: Production Strategies, Gel-Forming Properties, and Advances in Drug Delivery Systems
Abstract
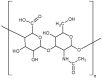
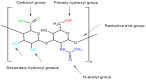
Hyaluronic acid (HA) is a naturally occurring glycosaminoglycan widely recognised for its biocompatibility, biodegradability, and unique viscoelastic properties. Its structural versatility enables the formation of hydrogels with tuneable physicochemical characteristics, making it a valuable biomaterial in drug delivery and regenerative medicine. This review outlines HA properties, gel-forming approaches, and modern medicine and bioengineering applications. It provides a comprehensive overview of advances in HA production strategies, including microbial fermentation, animal tissue extraction, and production in vitro. Particular attention is given to gel-forming mechanisms, emphasising physical and chemical crosslinking methods like carbodiimide crosslinking, radical polymerisation, and enzymatic crosslinking. Advances in HA-based drug delivery systems and applications of HA-based materials in tissue engineering are also discussed, focusing on HA-based hydrogels with conjugates and combinations with compounds like collagen, alginate, and chitosan.
Keywords: drug delivery; hyaluronic acid; hyaluronic-acid-based hydrogels; microbial hyaluronic acid production.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


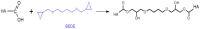






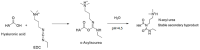



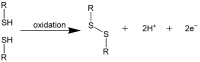
References
-
- Hirschberg J. Zentralblatt Für Praktische Augenheilkunde. Verlag von Veit & Comp.; Leibzig, Germany: 1880.
-
- Meyer K., Palmer J.W. The Polysaccharide of the Vitreous Humor. J. Biol. Chem. 1934;107:629–634. doi: 10.1016/S0021-9258(18)75338-6. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

