Gelatin/Cerium-Doped Bioactive Glass Composites for Enhancing Cellular Functions of Human Mesenchymal Stem Cells (hBMSCs)
- PMID: 40558726
- PMCID: PMC12192449
- DOI: 10.3390/gels11060425
Gelatin/Cerium-Doped Bioactive Glass Composites for Enhancing Cellular Functions of Human Mesenchymal Stem Cells (hBMSCs)
Abstract
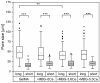
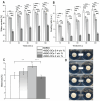
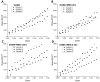
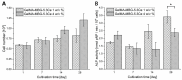
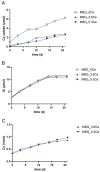
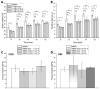
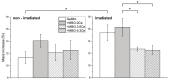
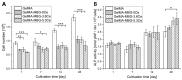
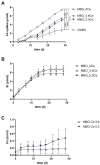
Delayed or non-healing of bone defects in an aging, multi-morbid population is still a medical challenge. Current replacement materials, like autografts, are limited. Thus, artificial substitutes from biodegradable polymers and bioactive glasses (BGs) are promising alternatives. Here, novel cerium-doped mesoporous BG microparticles (Ce-MBGs) with different cerium content were included in photocrosslinkable, methacrylated gelatin (GelMA) for promoting cellular functions of human mesenchymal stem cells (hBMSCs). The composites were studied for intrinsic morphology and Ce-MBGs distribution by scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDX). They were gravimetrically analyzed for swelling and stability, compressive modulus via Microsquisher® and bioactivity by Fluitest® calcium assay and inductively coupled plasma-optical emission spectrometry (ICP-OES), also determining silicon and cerium ion release. Finally, seeding, proliferation, and differentiation of hBMSCs was investigated. Ce-MBGs were evenly distributed within composites. The latter displayed a concentration-dependent but cerium-independent decrease in swelling, while mechanical properties were comparable. A MBG type-dependent bioactivity was shown, while an enhanced osteogenic differentiation of hBMSCs was achieved for Ce-MBG-composites and related to different ion release profiles. These findings show their strong potential in promoting bone regeneration. Still, future work is required, e.g., analyzing the expression of osteogenic genes, providing further evidence for the composites' osteogenic effect.
Keywords: cerium-doped mesoporous bioactive glasses; human mesenchymal stem cells; hydrogels; methacrylated gelatin; osteogenic differentiation.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures









References
LinkOut - more resources
Full Text Sources

