Multiplexing 3D Natural Scaffolds to Optimize the Repair and Regeneration of Chronic Diabetic Wounds
- PMID: 40558729
- PMCID: PMC12192242
- DOI: 10.3390/gels11060430
Multiplexing 3D Natural Scaffolds to Optimize the Repair and Regeneration of Chronic Diabetic Wounds
Erratum in
-
Correction: Moldovan et al. Multiplexing 3D Natural Scaffolds to Optimize the Repair and Regeneration of Chronic Diabetic Wounds. Gels 2025, 11, 430.Gels. 2025 Jul 16;11(7):549. doi: 10.3390/gels11070549. Gels. 2025. PMID: 40710726 Free PMC article.
Abstract
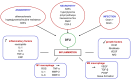
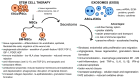
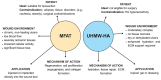
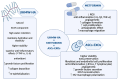
Diabetic foot ulcers (DFU) represent a major complication of diabetes mellitus, affecting millions of patients worldwide and leading to high morbidity and amputation risks. The impaired healing process in DFU is driven by vascular insufficiency, neuropathy, chronic inflammation, and infections. Conventional treatments, including blood sugar control, wound debridement, and standard dressings, have shown limited efficacy in achieving complete healing. Recent advancements have introduced novel therapeutic approaches such as stem cell therapy, exosome-based treatments, and bioengineered scaffolds to accelerate wound healing and tissue regeneration. Mesenchymal stem cells (MSCs), particularly adipose-derived stem cells (ASCs), exhibit anti-inflammatory, pro-angiogenic, and immunomodulatory properties, enhancing wound repair. Additionally, exosomes derived from ASCs have demonstrated the ability to promote fibroblast proliferation, regulate inflammation, and stimulate angiogenesis. The integration of bioengineered scaffolds, including hydrogels, hyaluronic acid (HA), or micro-fragmented adipose tissue (MFAT), offers improved drug delivery mechanisms and a controlled healing environment. These scaffolds have been successfully utilized to deliver stem cells, growth factors, antioxidants, anti-glycation end products, anti-inflammatory and anti-diabetic drugs, or antimicrobial agents, further improving DFU outcomes. This review highlights the potential of combining novel 3D scaffolds with anti-diabetic drugs to enhance DFU treatment, reduce amputation rates, and improve patients' quality of life. While promising, further clinical research is required to validate these emerging therapies and optimize their clinical application.
Keywords: 3D scaffolds; diabetic foot ulcer; drug-delivery; wound healing.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

