Nanoemulsion Hydrogel Delivery System of Hypericum perforatum L.: In Silico Design, In Vitro Antimicrobial-Toxicological Profiling, and In Vivo Wound-Healing Evaluation
- PMID: 40558730
- PMCID: PMC12192460
- DOI: 10.3390/gels11060431
Nanoemulsion Hydrogel Delivery System of Hypericum perforatum L.: In Silico Design, In Vitro Antimicrobial-Toxicological Profiling, and In Vivo Wound-Healing Evaluation
Abstract
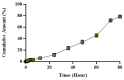
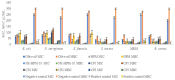
Hypericum perforatum L. (H.P.), a plant renowned for its wound-healing properties, was investigated for antioxidant/antimicrobial efficacy, toxicological safety, and in vivo wound-healing effects in this research to develop and characterize novel nanoemulsion hydrogel (NG) formulations. NG were prepared via emulsion diffusion-solvent evaporation and polymer hydration using Cremophor RH40 and Ultrez 21/30. A D-optimal design optimized oil/surfactant ratios, considering particle size, PDI, and drug loading. Antioxidant activity was tested via DPPH, ABTS+, and FRAP. Toxicological assessment followed HET-CAM (ICH-endorsed) and ICCVAM guidelines. The optimized NG-2 (NE-HPM-10 + U30 0.5%) demonstrated stable and pseudoplastic flow, with a particle size of 174.8 nm, PDI of 0.274, zeta potential of -23.3 mV, and 99.83% drug loading. Release followed the Korsmeyer-Peppas model. H.P. macerates/NEs showed potent antioxidant activity (DPPH IC50: 28.4 µg/mL; FRAP: 1.8 mmol, Fe2+/g: 0.3703 ± 0.041 mM TE/g). Antimicrobial effects against methicillin-resistant S. aureus (MIC: 12.5 µg/mL) and E. coli (MIC: 25 µg/mL) were significant. Stability studies showed no degradation. HET-CAM tests confirmed biocompatibility. Histopathology revealed accelerated re-epithelialization/collagen synthesis, with upregulated TGF-β1. The NG-2 formulation demonstrated robust antioxidant, antimicrobial, and wound-healing efficacy. Enhanced antibacterial activity and biocompatibility highlight its therapeutic potential. Clinical/pathological evaluations validated tissue regeneration without adverse effects, positioning H.P.-based nanoemulsions as promising for advanced wound care.
Keywords: Cremophor RH40; HET-CAM; Hypericum perforatum L.; antimicrobial activity; antioxidant activity; drug delivery systems; hydrogel; in silico modeling; nanoemulsion; wound healing.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures












Similar articles
-
Newly designed curcumin-loaded hybrid nanoparticles: a multifunctional strategy for combating oxidative stress, inflammation, and infections to accelerate wound healing and tissue regeneration.BMC Biotechnol. 2025 Jun 19;25(1):49. doi: 10.1186/s12896-025-00989-z. BMC Biotechnol. 2025. PMID: 40537758 Free PMC article.
-
Synthesis of a retro-GFOGER Adamantane-Based Collagen Mimetic Peptide Imbibed in a Hyaluronic Acid Hydrogel for Enhanced Wound Healing.ACS Appl Bio Mater. 2025 Jun 16;8(6):4657-4672. doi: 10.1021/acsabm.4c01895. Epub 2025 Feb 19. ACS Appl Bio Mater. 2025. PMID: 39970309 Free PMC article.
-
Innovative Hydrogel Formulation Combining Phycocyanin and Probiotic for Enhancing Skin Regeneration and Accelerated Wound Healing: A Preclinical Investigation in Wistar Rats.Probiotics Antimicrob Proteins. 2025 Jul 12. doi: 10.1007/s12602-025-10635-x. Online ahead of print. Probiotics Antimicrob Proteins. 2025. PMID: 40650833
-
Hydrogel dressings for venous leg ulcers.Cochrane Database Syst Rev. 2022 Aug 5;8(8):CD010738. doi: 10.1002/14651858.CD010738.pub2. Cochrane Database Syst Rev. 2022. PMID: 35930364 Free PMC article.
-
Topical antimicrobial agents for treating foot ulcers in people with diabetes.Cochrane Database Syst Rev. 2017 Jun 14;6(6):CD011038. doi: 10.1002/14651858.CD011038.pub2. Cochrane Database Syst Rev. 2017. PMID: 28613416 Free PMC article.
References
-
- Tavasli A., Navidfar A., Inangil D., Inangil G., Aslan I., Trabzon L. Hydrophilic sol-gel TiO2 coating on procaine-loaded injector needle for painless clinical treatments. Colloids Surf. A Physicochem. Eng. Asp. 2024;687:133556. doi: 10.1016/j.colsurfa.2024.133556. - DOI
-
- Srivastava M.K. Advances in Mergers and Acquisitions. Volume 23. Emerald Publishing Limited; Bingley, UK: 2024. Strategic Choices Between Alliances and Acquisitions: What Do We Know and Where Do We Go from Here? pp. 123–131.
LinkOut - more resources
Full Text Sources
Miscellaneous

