Effects of Melatonin-Loaded Poly(N-vinylcaprolactam) Transdermal Gel on Sleep Quality
- PMID: 40558734
- PMCID: PMC12191773
- DOI: 10.3390/gels11060435
Effects of Melatonin-Loaded Poly(N-vinylcaprolactam) Transdermal Gel on Sleep Quality
Abstract
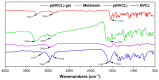
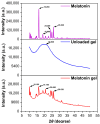
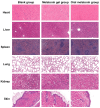
The rapid pace of modern life has contributed to a significant decline in sleep quality, which has become an urgent global public health issue. Melatonin, an endogenous hormone that regulates circadian rhythms, is vital in maintaining normal sleep cycles. While oral melatonin supplementation is widely used, transdermal delivery systems present advantages that include the avoidance of first-pass metabolism effects and enhanced bioavailability. In this study, a novel melatonin transdermal delivery system was successfully developed using a thermosensitive poly(N-vinylcaprolactam) [p(NVCL)]-based carrier. The p(NVCL) polymer was synthesized through free radical polymerization and characterized for its structural properties and phase transition temperature, in alignment with skin surface conditions. Orthogonal optimization experiments identified 3% azone, 3% menthol, and 4% borneol as the optimal enhancer combination for enhanced transdermal absorption. The formulation demonstrated exceptional melatonin loading characteristics with high encapsulation efficiency and stable physicochemical properties, including an appropriate pH and optimal moisture content. Comprehensive in vivo evaluation using normal mouse models revealed significant sleep quality improvements, specifically a shortened sleep latency and extended non-rapid eye movement sleep duration, with elevated serum melatonin and serotonin levels. Safety assessments including histopathological examination, biochemical analysis, and 28-day continuous administration studies confirmed excellent biocompatibility with no adverse reactions or systemic toxicity. Near-infrared fluorescence imaging provided direct evidence of enhanced transdermal absorption and superior biodistribution compared to oral administration. These findings indicate that the p(NVCL)-based melatonin transdermal gel system offers a safe, effective and convenient non-prescription option for sleep regulation, with promising potential for clinical translation as a consumer sleep aid.
Keywords: melatonin; poly(N-vinylcaprolactam); sleep quality; transdermal gel; transdermal permeation enhancers.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Cavaillès C., Dintica C.S., Habes M., Leng Y., Carnethon M., Yaffe K. Sleep Patterns in Midlife and Brain Age. Alzheimer’s Dement. 2024;20:e085643. doi: 10.1002/alz.085643. - DOI
-
- Carnes A., Piñol-Ripoll G., Ariza M., Cano N., Segura B., Junqué C., Garoellda M. Poor sleep quality as a potential trigger for cognitive deficits. Alzheimer’s Dement. 2024;20:e087261. doi: 10.1002/alz.087261. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

