Multi-Organ Adverse Reaction to Two Hypomethylating Agents: A Challenge in High-Risk Myelodysplastic Syndrome Treatment
- PMID: 40558807
- PMCID: PMC12193438
- DOI: 10.3390/hematolrep17030029
Multi-Organ Adverse Reaction to Two Hypomethylating Agents: A Challenge in High-Risk Myelodysplastic Syndrome Treatment
Abstract
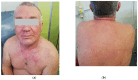
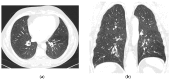
Background and Clinical Significance: Intermediate- to high-risk Myelodysplastic Syndrome (MDS), according to the Revised International Prognostic Scoring System (IPSS-M), confers a high risk of progression into acute myeloid leukemia. Treatment with hypomethylating agents, including azacitidine and decitabine, represents the current standard of care. In eligible patients, hypomethylating agents are used as a bridge for allogeneic stem cell transplantation, currently the only curative approach in these malignancies. The most common side effects of hypomethylating agents are myelosuppression, cutaneous injection site reactions (when azacitidine is given subcutaneously), and gastrointestinal symptoms. Uncommon, disabling, and long-lasting side effects represent a threat to effective treatment in this group of patients. Case Presentation: We describe the case of a 49-year-old male patient with IPSS-M intermediate-risk MDS, intended to receive first-line treatment with azacitidine followed by allogeneic stem cell transplantation. The first, late-onset azacitidine reaction was observed 48 h after the first exposure, with cutaneous and respiratory toxicity, followed by the late-onset recurrence of symptoms after azacitidine withdrawal and decitabine introduction. Conclusions: This case highlights atypical, disabling, and long-lasting drug reactions to two hypomethylating agents, with the persistence of hypersensitivity manifestations months after medication withdrawal.
Keywords: adverse drug reaction; azacitidine; decitabine; drug-induced cutaneous reaction; drug-induced pulmonary toxicity; hemato-oncological patients; hypersensitivity; hypomethylating agent; myelodysplastic syndrome.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

