Histone H3 Lysine 9 Acetylation Plays a Role in Adipogenesis of Periodontal Ligament-Derived Stem Cells
- PMID: 40558826
- PMCID: PMC12192146
- DOI: 10.3390/epigenomes9020015
Histone H3 Lysine 9 Acetylation Plays a Role in Adipogenesis of Periodontal Ligament-Derived Stem Cells
Abstract
Background: The epigenetic regulation of adipogenic differentiation in dental stem cells (DSCs) remains poorly understood, as research has prioritized osteogenic differentiation for dental applications. However, elucidating these mechanisms could enable novel regenerative strategies for soft tissue engineering. Periodontal ligament stem cells (PDLSCs) exhibit notable adipogenic potential, possibly linked to histone 3 acetylation at lysine 9 (H3K9ac); however, the mechanistic role of this modification remains unclear.
Methods: To address this gap, we investigated how histone deacetylase inhibitors (HDACis)-valproic acid (VPA, 8 mM) and trichostatin A (TSA, 100 nM)-modulate H3K9ac dynamics, adipogenic gene expression (C/EBPβ and PPARγ-2), and chromatin remodeling during PDLSCs differentiation. Techniques used included quantitative PCR (qPCR), lipid droplet analysis, and chromatin immunoprecipitation followed by qPCR (ChIP-qPCR).
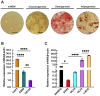
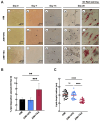
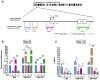
Results: TSA-treated cells exhibited increased lipid deposition with smaller lipid droplets compared to VPA-treated cells. Global H3K9ac levels correlated positively with adipogenic progression. VPA induced early upregulation of C/EBPβ and PPARγ-2 (day 7), whereas TSA triggered a delayed but stronger PPARγ-2 expression. ChIP-qPCR analysis revealed significant H3K9ac enrichment at the PPARγ-2 promoter in TSA-treated cells, indicating enhanced chromatin accessibility.
Conclusions: These findings demonstrate that H3K9ac-mediated epigenetic remodeling plays a critical role in the adipogenic differentiation of PDLSCs and identifies TSA as a potential tool for modulating this process.
Keywords: adipogenic differentiation; dental stem cells; histone H3 lysine 9 acetylation; histone deacetylase inhibitors; periodontal ligament stem cells.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Inhibition of histone deacetylases class I improves adipogenic differentiation of human periodontal ligament cells.Cell Mol Biol (Noisy-le-grand). 2024 May 27;70(5):40-47. doi: 10.14715/cmb/2024.70.5.7. Cell Mol Biol (Noisy-le-grand). 2024. PMID: 38814236
-
JIB-04 improves PPARγ expression and enhances adipogenic differentiation efficiency in human umbilical cord mesenchymal stem cells.Biochem Biophys Rep. 2025 Jul 9;43:102142. doi: 10.1016/j.bbrep.2025.102142. eCollection 2025 Sep. Biochem Biophys Rep. 2025. PMID: 40688503 Free PMC article.
-
14-3-3ζ allows for adipogenesis by modulating chromatin accessibility during the early stages of adipocyte differentiation.Mol Metab. 2025 Jul;97:102159. doi: 10.1016/j.molmet.2025.102159. Epub 2025 Apr 28. Mol Metab. 2025. PMID: 40306359 Free PMC article.
-
Guided tissue regeneration for periodontal infra-bony defects.Cochrane Database Syst Rev. 2006 Apr 19;(2):CD001724. doi: 10.1002/14651858.CD001724.pub2. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2019 May 29;5:CD001724. doi: 10.1002/14651858.CD001724.pub3. PMID: 16625546 Updated.
-
Full-mouth treatment modalities (within 24 hours) for periodontitis in adults.Cochrane Database Syst Rev. 2022 Jun 28;6(6):CD004622. doi: 10.1002/14651858.CD004622.pub4. Cochrane Database Syst Rev. 2022. PMID: 35763286 Free PMC article.
References
-
- Darwish N.M., Gouda W., Almutairi S.M., Elshikh M.S., Morcos G.N.B. PPARG Expression Patterns and Correlations in Obesity. J. King Saud Univ. Sci. 2022;34:102116. doi: 10.1016/j.jksus.2022.102116. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

