A Modular Biomimetic Preclinical Platform to Elucidate the Interaction Between Cancer Cells and the Bone Metastatic Niche
- PMID: 40558906
- PMCID: PMC12194721
- DOI: 10.3390/jfb16060220
A Modular Biomimetic Preclinical Platform to Elucidate the Interaction Between Cancer Cells and the Bone Metastatic Niche
Abstract
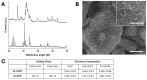
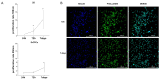
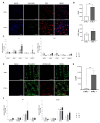
Breast cancer (BC) frequently metastasizes to bone, leading to poor patient prognosis. The infiltration of cancer cells in bone impairs its homeostasis, triggering a pathological interaction between tumors and resident cells. Preclinical models able to mimic the bone microenvironment are needed to advance translational findings on BC mechanisms and treatments. We designed strontium-doped calcium phosphate cement to be employed for culturing cancer and bone cells and developed an in vitro bone metastasis model. The platform was established step by step, starting with the monoculture of cancer cells, mature osteoblasts (OBs) differentiated from mesenchymal stem cells, and mature osteoclasts (OCs) differentiated from Peripheral Blood Mononuclear Cells. The model was implemented with the co-culture of cancer cells with OBs or OCs, or the co-culture of OBs and OCs, allowing us to discriminate the interaction between the actors of the bone metastatic niche. The biomimetic material was further challenged with bone metastasis patient-derived material, showing good versatility and biocompatibility, suggesting its potential use as bone substitute. Overall, we developed a bone-mimicking model able to reproduce reciprocal interactions between cancer and bone cells in a biomimetic environment suitable for studying the biomolecular determinants of bone metastasis and, in the future, as a drug efficacy platform.
Keywords: bone metastasis; breast cancer; osteoblasts; osteoclasts; patient-derived explants; preclinical platform; tricalcium phosphate cements.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
Bisphosphonates and other bone agents for breast cancer.Cochrane Database Syst Rev. 2017 Oct 30;10(10):CD003474. doi: 10.1002/14651858.CD003474.pub4. Cochrane Database Syst Rev. 2017. PMID: 29082518 Free PMC article.
-
A systematic review and economic evaluation of epoetin alpha, epoetin beta and darbepoetin alpha in anaemia associated with cancer, especially that attributable to cancer treatment.Health Technol Assess. 2007 Apr;11(13):1-202, iii-iv. doi: 10.3310/hta11130. Health Technol Assess. 2007. PMID: 17408534
-
Carbamazepine versus phenytoin monotherapy for epilepsy: an individual participant data review.Cochrane Database Syst Rev. 2017 Feb 27;2(2):CD001911. doi: 10.1002/14651858.CD001911.pub3. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2019 Jul 18;7:CD001911. doi: 10.1002/14651858.CD001911.pub4. PMID: 28240353 Free PMC article. Updated.
-
Treatments for seizures in catamenial (menstrual-related) epilepsy.Cochrane Database Syst Rev. 2021 Sep 16;9(9):CD013225. doi: 10.1002/14651858.CD013225.pub3. Cochrane Database Syst Rev. 2021. PMID: 34528245 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

