Gut-Microbiome Signatures Predicting Response to Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer: A Systematic Review
- PMID: 40559436
- PMCID: PMC12195113
- DOI: 10.3390/metabo15060412
Gut-Microbiome Signatures Predicting Response to Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer: A Systematic Review
Abstract
Background and objectives: Rectal cancer management increasingly relies on watch-and-wait strategies after neoadjuvant chemoradiotherapy (nCRT). Accurate, non-invasive prediction of pathological complete response (pCR) remains elusive. Emerging evidence suggests that gut-microbiome composition modulates radio-chemosensitivity. We systematically reviewed primary studies that correlated baseline or on-treatment gut-microbiome features with nCRT response in locally advanced rectal cancer (LARC).
Methods: MEDLINE, Embase and PubMed were searched from inception to 30 April 2025. Eligibility required (i) prospective or retrospective human studies of LARC, (ii) faecal or mucosal microbiome profiling by 16S, metagenomics, or metatranscriptomics, and (iii) response assessment using tumour-regression grade or pCR. Narrative synthesis and random-effects proportion meta-analysis were performed where data were homogeneous.
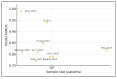
Results: Twelve studies (n = 1354 unique patients, median sample = 73, range 22-735) met inclusion. Four independent machine-learning models achieved an Area Under the Receiver Operating Characteristic curve AUROC ≥ 0.85 for pCR prediction. Consistently enriched taxa in responders included Lachnospiraceae bacterium, Blautia wexlerae, Roseburia spp., and Intestinimonas butyriciproducens. Non-responders showed over-representation of Fusobacterium nucleatum, Bacteroides fragilis, and Prevotella spp. Two studies linked butyrate-producing modules to radiosensitivity, whereas nucleotide-biosynthesis pathways conferred resistance. Pooled pCR rate in patients with a "butyrate-rich" baseline profile was 44% (95% CI 35-54) versus 21% (95% CI 15-29) in controls (I2 = 18%).
Conclusions: Despite heterogeneity, convergent functional and taxonomic signals underpin a microbiome-based radiosensitivity axis in LARC. Multi-centre validation cohorts and intervention trials manipulating these taxa, such as prebiotics or live-biotherapeutics, are warranted before clinical deployment.
Keywords: chemoradiotherapy; gut microbiome; metagenomics; pathologic complete response; predictive biomarker; rectal cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Response prediction for neoadjuvant treatment in locally advanced rectal cancer patients-improvement in decision-making: A systematic review.Eur J Surg Oncol. 2025 Jul;51(7):109463. doi: 10.1016/j.ejso.2024.109463. Epub 2024 Nov 15. Eur J Surg Oncol. 2025. PMID: 39562260
-
Relationships between the Microbiome and Response to Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer.Cancer Res Treat. 2025 Jul;57(3):840-851. doi: 10.4143/crt.2024.521. Epub 2024 Dec 16. Cancer Res Treat. 2025. PMID: 39701092 Free PMC article.
-
Magnetic resonance perfusion for differentiating low-grade from high-grade gliomas at first presentation.Cochrane Database Syst Rev. 2018 Jan 22;1(1):CD011551. doi: 10.1002/14651858.CD011551.pub2. Cochrane Database Syst Rev. 2018. PMID: 29357120 Free PMC article.
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article.
-
Comparison of the efficacy of neoadjuvant chemotherapy and neoadjuvant chemoradiotherapy in locally advanced rectal cancer patients: meta-analysis of randomized controlled trials.Int J Surg. 2025 Mar 1;111(3):2686-2696. doi: 10.1097/JS9.0000000000002262. Int J Surg. 2025. PMID: 39878151
References
-
- Zhang X., Ding R., Li J., Wu T., Shen Z., Li S., Zhang Y., Dong C., Shang Z., Zhou H., et al. Efficacy and safety of the “watch-and-wait” approach for rectal cancer with clinical complete response after neoadjuvant chemoradiotherapy: A meta-analysis. Surg. Endosc. 2022;36:2233–2244. doi: 10.1007/s00464-021-08932-x. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous