Towards Automated Testing of Kynurenine for Point-of-Care Metabolomics
- PMID: 40559444
- PMCID: PMC12196141
- DOI: 10.3390/mps8030056
Towards Automated Testing of Kynurenine for Point-of-Care Metabolomics
Abstract
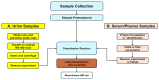
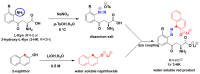
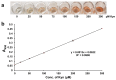
Our objective was to develop a simple, low-cost colorimetric assay to detect kynurenine (L-Kyn) in human biofluids, that would be compatible with a point-of-care (POC) system being developed in our lab. Elevated L-Kyn is associated with many pathological conditions. However, current detection methods are expensive, time-consuming, and unsuitable for resource-limited settings. Existing colorimetric L-Kyn assays lack specificity, require unusual reagents, or lack sensitivity, hindering their practical application. Here we report a two-step diazotization-based colorimetric assay that produces a red chromophore upon reaction with L-Kyn. To reduce background interference, we used dilution and anion exchange chromatography for urine samples and acid precipitation for serum samples. The assay detected 5-300 μM L-Kyn in urine (lower limit of detection (LLOD) 1.34 μM) and 5-125 μM L-Kyn in serum (LLOD 1.24 μM). Correlation studies achieved strong linearity (R2 = 0.98 for spiked urine, 0.99 for spiked serum) and were highly correlated (>0.95) to liquid chromatography tandem mass spectrometry (LC-MS/MS) concentrations. Bland-Altman analysis confirmed agreement between L-Kyn assay and LC-MS/MS methods. To our knowledge, this is the first application of a diazotization reaction for L-Kyn quantification at physiologically relevant levels. The assay is now being ported to a low-cost, automated POC biosensor platform.
Keywords: LC-MS/MS; chemical assay; diazotization; kynurenine; serum; urine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

