M72 Fusion Proteins in Nanocapsules Enhance BCG Efficacy Against Bovine Tuberculosis in a Mouse Model
- PMID: 40559600
- PMCID: PMC12195942
- DOI: 10.3390/pathogens14060592
M72 Fusion Proteins in Nanocapsules Enhance BCG Efficacy Against Bovine Tuberculosis in a Mouse Model
Abstract
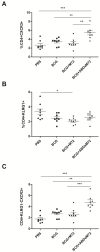
Mycobacterium bovis is the causative pathogen of bovine tuberculosis (bTB), a disease that affects cattle and other mammals, including humans. Currently, there is no efficient vaccine against bTB, underscoring the need for novel immunization strategies. The M72 fusion protein, composed of three polypeptides derived from Mycobacterium tuberculosis and M. bovis, has demonstrated protective efficacy against M. tuberculosis in clinical trials when combined with the AS01E adjuvant. Given the established efficacy of nanocapsule formulations as vaccine delivery systems, this study evaluated a novel immunization strategy combining BCG with either full-length M72 or a truncated M72 fused to a streptococcal albumin-binding domain (ABDsM72). Both antigens were encapsulated in chitosan/alginate nanocapsules and assessed in a murine M. bovis challenge model. Priming with BCG followed by an M72 boost significantly improved splenic protection compared to BCG alone, but it did not enhance pulmonary protection. Notably, boosting with ABDsM72 further increased the proportion of CD4+KLRG1-CXCR3+ T cells in the lungs of M. bovis-challenged mice, a key correlate of protective immunity. These findings demonstrate that chitosan/alginate-encapsulated antigens enhance BCG-induced immunity, supporting their potential as next-generation vaccine candidates for bTB control.
Keywords: M72; Mycobacterium bovis; bovine tuberculosis; chitosan nanocapsules; mice; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

