Role of Compensatory miRNA Networks in Cognitive Recovery from Heart Failure
- PMID: 40559623
- PMCID: PMC12196295
- DOI: 10.3390/ncrna11030045
Role of Compensatory miRNA Networks in Cognitive Recovery from Heart Failure
Abstract
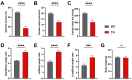
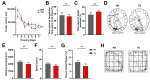
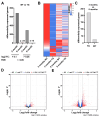
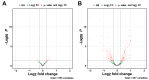
Background: Heart failure (HF) is associated with an increased risk of cognitive impairment and hippocampal dysfunction, yet the underlying molecular mechanisms remain poorly understood. This study aims to investigate the role of microRNA (miRNA) networks in hippocampus-dependent memory recovery in a mouse model of HF. Methods: CaMKIIδC transgenic (TG) mice, a model for HF, were used to assess hippocampal function at 3 and 6 months of age. Memory performance was evaluated using hippocampus-dependent behavioral tasks. Small RNA sequencing was performed to analyze hippocampal miRNA expression profiles across both time points. Bioinformatic analyses identified miRNAs that potentially regulate genes previously implicated in HF-induced cognitive impairment. Results: We have previously shown that at 3 months of age, CaMKIIδC TG mice exhibited significant memory deficits associated with dysregulated hippocampal gene expression. In this study, we showed that these impairments, memory impairment and hippocampal gene expression, were no longer detectable at 6 months, despite persistent cardiac dysfunction. However, small RNA sequencing revealed a dynamic shift in hippocampal miRNA expression, identifying 27 miRNAs as "compensatory miRs" that targeted 73% of the transcripts dysregulated at 3 months but reinstated by 6 months. Notably, miR-181a-5p emerged as a central regulatory hub, with its downregulation coinciding with restored memory function. Conclusions: These findings suggest that miRNA networks contribute to the restoration of hippocampal function in HF despite continued cardiac pathology and provide an important compensatory mechanism towards memory impairment. A better understanding of these compensatory miRNA mechanisms may provide novel therapeutic targets for managing HF-related cognitive dysfunction.
Keywords: Alzheimer; MicroRNA; cognitive impairment; heart failure; hippocampal function; memory recovery; transcriptional homeostasis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Selegiline for Alzheimer's disease.Cochrane Database Syst Rev. 2003;(1):CD000442. doi: 10.1002/14651858.CD000442. Cochrane Database Syst Rev. 2003. PMID: 12535396
-
Occupational therapy for cognitive impairment in stroke patients.Cochrane Database Syst Rev. 2022 Mar 29;3(3):CD006430. doi: 10.1002/14651858.CD006430.pub3. Cochrane Database Syst Rev. 2022. PMID: 35349186 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Withdrawal or continuation of cholinesterase inhibitors or memantine or both, in people with dementia.Cochrane Database Syst Rev. 2021 Feb 3;2(2):CD009081. doi: 10.1002/14651858.CD009081.pub2. Cochrane Database Syst Rev. 2021. PMID: 35608903 Free PMC article.
References
-
- Carpenter A.E., Jones T.R., Lamprecht M.R., Clarke C., Kang I.H., Friman O., Guertin D.A., Chang J.H., Lindquist R.A., Moffat J., et al. CellProfiler: Image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006;7:R100. doi: 10.1186/gb-2006-7-10-r100. - DOI - PMC - PubMed
-
- Forman D.E., Daniels K.M., Cahalin L.P., Zavin A., Allsup K., Cao P., Santhanam M., Joseph J., Arena R., Lazzari A., et al. Analysis of skeletal muscle gene expression patterns and the impact of functional capacity in patients with systolic heart failure. J. Card. Fail. 2014;20:422–430. doi: 10.1016/j.cardfail.2014.03.007. - DOI - PMC - PubMed
-
- Rademaker M.T., Pilbrow A.P., Ellmers L.J., Palmer S.C., Davidson T., Mbikou P., Scott N.J.A., Permina E., Charles C.J., Endre Z.H., et al. Acute Decompensated Heart Failure and the Kidney: Physiological, Histological and Transcriptomic Responses to Development and Recovery. J. Am. Heart Assoc. 2021;10:e021312. doi: 10.1161/JAHA.121.021312. - DOI - PMC - PubMed
Grants and funding
- priority program 1738, SFB1286 and GRK2824, Excellence Strategy - EXC 2067/1 390729940/German Research Foundation
- EPI-3E/The EU Joint Programme- Neurodegenerative Diseases (JPND)
- miRassay (16LW0055)/German Federal Ministry of Science and Education
- DZHK Innovation Cluster Brain and Heart Interfaces/DZHK
- Heisenberg Stipend/German Resarch Foundation
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

