Potential of Marine Bacterial Metalloprotease A69 in the Preparation of Antarctic Krill Peptides with Multi-Bioactivities
- PMID: 40559635
- PMCID: PMC12194120
- DOI: 10.3390/md23060226
Potential of Marine Bacterial Metalloprotease A69 in the Preparation of Antarctic Krill Peptides with Multi-Bioactivities
Abstract
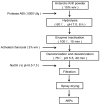
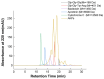
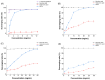
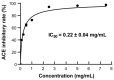
Antarctic krill (Euphausia superba) is a nutrient-rich marine resource. Although several terrestrial proteases have been used to prepare Antarctic krill peptides (AKPs), there has been no report on the preparation of AKPs using a marine protease. Here, marine bacterial protease A69 was used to prepare AKPs with multi-bioactivities. Through optimizing hydrolysis parameters, we established a process for AKPs preparation by hydrolyzing Antarctic krill powder with A69. In the prepared AKPs, peptides less than 3000 Da and 1000 Da accounted for 99.23% and 88.37%, respectively. The scavenging ratios of the AKPs to ABTS+, DPPH· and ·OH reached 93.23 ± 0.09%, 99.90 ± 0.15%, and 93.90 ± 0.47%, respectively. The AKPs also had high angiotensin-converting enzyme (ACE)-inhibitory activity, with an IC50 of 0.22 ± 0.04 mg/mL. At 40 mg/mL, the AKPs inhibited α-glucosidase and dipeptidyl peptidase IV (DPP-IV) activities by 7.18% and 13.62%, respectively, and displayed antibacterial activity to Escherichia coli. Moreover, 14 antioxidant peptides, 24 ACE-inhibitory peptides, 2 α-glucosidase-inhibitory peptides, and 10 DPP-Ⅳ-inhibitory peptides were identified from the AKPs. These results demonstrate that the prepared AKPs contain diverse bioactive peptides and have multi-bioactivities. This study indicates that marine bacterial protease A69 has promising application potential in preparing AKPs with multi-bioactivities.
Keywords: Antarctic krill peptides; angiotensin-converting enzyme (ACE)-inhibitory activity; antibacterial activity; antioxidant activity; dipeptidyl peptidase IV (DPP-IV)-inhibitory activity; marine bacterial protease A69; α-glucosidase-inhibitory activity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

