The Role of a Conserved Arg-Asp Pair in the Structure and Function of Tetanus Neurotoxin
- PMID: 40559851
- PMCID: PMC12197568
- DOI: 10.3390/toxins17060273
The Role of a Conserved Arg-Asp Pair in the Structure and Function of Tetanus Neurotoxin
Abstract
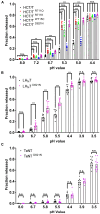
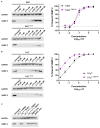
Tetanus, a severe and life-threatening illness caused by Clostridium tetani, produces symptoms such as muscle spasms, muscle stiffness and seizures caused by the production of tetanus neurotoxin (TeNT). TeNT causes spastic paralysis through the inhibition of neurotransmission in spinal inhibitory interneurons. This is achieved, in part, through pH-triggered membrane insertion of the translocation (HCT) domain, which delivers the catalytic light-chain (LC) domain to the cytosol. While the function of HCT is well defined, the mechanism by which it accomplishes this task is largely unknown. Based on the crystal structure of tetanus neurotoxin, we identified potential polar interactions between arginine 711, tryptophan 715 and aspartate 821 that appear to be evolutionarily conserved across the clostridial neurotoxin family. We show that the disruption of the Asp-Arg pair in a beltless HCT variant (bHCT) results in changes in thermal stability without significant alterations to the overall secondary structure. ANS (1-anilino-8-napthalene sulfonate) binding studies, in conjunction with liposome permeabilization assays, demonstrate that mutations at R711 or D821 trigger interactions with the membrane at higher pH values compared to wildtype bHCT. Interestingly, we show that the introduction of the D821N mutation into LHNT (LC-HCT only), but not the holotoxin, resulted in the increased cleavage of VAMP 2 in cortical neurons relative to the wildtype protein. This suggests that, as observed for botulinum toxin A, the receptor-binding domain is not necessary for LC translocation but rather helps determine the pH threshold of membrane insertion. The mutation of W715 did not result in detectable changes in the activity of either bHCT or the holotoxin, suggesting that it plays only a minor role in stabilizing the structure of the toxin. We conclude that the protonation of D821 at low pH disrupts interactions with R711 and W715, helping to drive the conformational refolding of HCT needed for membrane insertion and the subsequent translocation of the LC.
Keywords: clostridial neurotoxins; pH trigger; pore formation; tetanus toxin; translocation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Expression of Recombinant Clostridial Neurotoxin by C. tetani.Microorganisms. 2024 Dec 17;12(12):2611. doi: 10.3390/microorganisms12122611. Microorganisms. 2024. PMID: 39770813 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.Health Technol Assess. 2008 Jun;12(28):iii-iv, ix-95. doi: 10.3310/hta12280. Health Technol Assess. 2008. PMID: 18547499
References
-
- Udwadia F.E. Tetanus. Oxford University Press; Bombay, India: 1994. pp. 1–172.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

