HMGB1 as a Key Mediator in Malignant Mesothelioma and a Potential Target for Asbestos-Related Cancer Therapy
- PMID: 40559922
- PMCID: PMC12197314
- DOI: 10.3390/toxics13060448
HMGB1 as a Key Mediator in Malignant Mesothelioma and a Potential Target for Asbestos-Related Cancer Therapy
Abstract
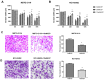
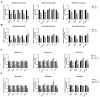
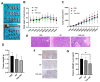
Malignant mesothelioma (MM) is a highly aggressive cancer strongly associated with asbestos exposure, and accumulating evidence suggests that high mobility group box 1 (HMGB1) plays a central role in its pathogenesis. Our in vitro and in vivo experiments revealed that HMGB1 was highly expressed in MM. Both genetic and pharmacological inhibition of HMGB1 markedly suppressed MM cell viability, migration, and invasion, while inducing G1-phase cell cycle arrest and enhancing apoptosis. Interestingly, the inhibition of Toll-like receptor 4 (TLR4), achieved through both siRNA and TAK-242 treatment, not only suppressed tumor-promoting signals but also reduced HMGB1 expression, suggesting a self-amplifying HMGB1-TLR4 loop. Mechanistically, in vitro experiments indicated that suppression of HMGB1 and TLR4 was associated with decreased activation of NF-κB, AKT, and ERK pathways, which are involved in regulating MM cell survival and motility. In xenograft models, treatment with ethyl pyruvate (EP) and TAK-242 significantly suppressed tumor growth and HMGB1 expression, reinforcing their therapeutic potential. Given HMGB1's influence on both tumor cell behavior and the immune microenvironment, targeting the HMGB1-TLR4 axis may not only provide a novel therapeutic strategy for MM but also offer insights into the mechanisms underlying asbestos-induced tumorigenesis, potentially guiding future prevention and intervention strategies in asbestos-exposed populations.
Keywords: HMGB1; TLR4; asbestos-related tumors; malignant mesothelioma; tumor progression.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
Grants and funding
- 202413023017/National Innovation Training Program for College Students
- 2020KY100/Medical Science and Technology Project of Zhejiang Province
- YS2022011/Institutional Special Program for Healthy Zhejiang
- First Class, Category A/Key Discipline of Zhejiang Province in Public Health and Preventive Medicine
LinkOut - more resources
Full Text Sources
Miscellaneous

