The Metal Oxidation State in Cu, CuO, and Cu2O Nanoparticles Plays a Key Role in Toxicity to Sea Urchin Arbacia lixula, Paracentrotus lividus, and Sphaerechinus granularis Embryos
- PMID: 40559942
- PMCID: PMC12197137
- DOI: 10.3390/toxics13060469
The Metal Oxidation State in Cu, CuO, and Cu2O Nanoparticles Plays a Key Role in Toxicity to Sea Urchin Arbacia lixula, Paracentrotus lividus, and Sphaerechinus granularis Embryos
Abstract
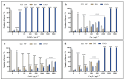
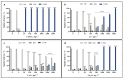
Copper-based nanoparticles (as Cu2O) are a key component in marine antifouling paints and, as coatings degrade, release nanoparticles that can affect a wide range of non-target organisms. This study investigates the impact of Cu2O nanoparticles on the early development of urchins Arbacia lixula, Paracentrotus lividus and Sphaerechinus granularis, and benchmarks their toxicity against similarly sized Cu and CuO nanoparticles and ionic copper. Concentration-dependent toxicity was noted for all forms of copper at concentrations in the 1 to 5000 µg L-1 range. EC50 values after Cu2O exposure indicated that A. lixula (99 µg L-1) was generally more sensitive than the other two species, with EC50 values of 371 µg L-1 and 606 µg L-1 noted for S. granularis and P. lividus, respectively. The same trend across species was noted for both Cu and CuO, although these nanoparticles generally showed higher EC50 values, indicating lower toxicity compared to Cu2O. LC50 values qualitatively parallel the corresponding EC50 values, with Cu2O consistently the most toxic, while Cu was less harmful, and CuO did not reach LC50 at any concentration. Again, greatest lethality was noted in A. lixula. While copper ion release from Cu was much greater than from CuO and Cu2O, the latter showed similar or greater toxicity to developing embryos compared to Cu. This indicates that copper ions are not the sole driver of toxicity of Cu2O, but there may also be a contribution derived from Cu2O redox activity within cells or at membranes that negatively impact oxidative stress defence mechanisms and metabolic pathways.
Keywords: antifouling; copper; developmental defect; embryogenesis; marine; paint; skeletogenesis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in adults and children aged 12 years and over.Health Technol Assess. 2008 May;12(19):iii-iv, 1-360. doi: 10.3310/hta12190. Health Technol Assess. 2008. PMID: 18485271
-
Antidepressants for pain management in adults with chronic pain: a network meta-analysis.Health Technol Assess. 2024 Oct;28(62):1-155. doi: 10.3310/MKRT2948. Health Technol Assess. 2024. PMID: 39367772 Free PMC article.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Nutritional interventions for survivors of childhood cancer.Cochrane Database Syst Rev. 2016 Aug 22;2016(8):CD009678. doi: 10.1002/14651858.CD009678.pub2. Cochrane Database Syst Rev. 2016. PMID: 27545902 Free PMC article.
References
-
- Raffi M., Mehrwan S., Bhatti T.M., Akhter J.I., Hameed A., Yawar W., Ul Hasan M.M. Investigations into the antibacterial behavior of copper nanoparticles against Escherichia coli. Ann. Microbiol. 2010;60:75–80. doi: 10.1007/s13213-010-0015-6. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

