Modification of Biochar Catalyst Using Copper for Enhanced Catalytic Oxidation of VOCs
- PMID: 40559976
- PMCID: PMC12197257
- DOI: 10.3390/toxics13060503
Modification of Biochar Catalyst Using Copper for Enhanced Catalytic Oxidation of VOCs
Abstract
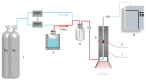
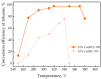
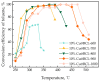
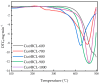
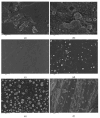
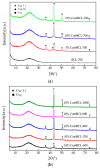
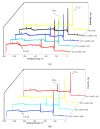
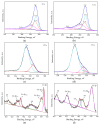
Recently, research has increasingly focused on the introduction of non-precious metals and developing highly stable carriers to enhance catalyst performance. In this study, we successfully synthesized copper (Cu)-modified biochar catalysts utilizing a sequential approach involving enzymatic treatment, liquid impregnation, and activation processes, which effectively enhanced the dispersion and introduction efficiency of Cu onto the biochar, thereby reducing the requisite Cu loading while maintaining high catalytic activity. The experimental results showed that the toluene degradation of 10%Cu@BCL was three times higher than that of unmodified activated carbon (AC) at 290 °C. A more uniform distribution of Cu was obtained by the enzymatic and activation treatments, optimizing the catalyst's structural properties and reducing the amount of Cu on the biochar. Moreover, the transformation between various oxidation states of Cu (from Cu0/Cu(I) to Cu(II)) facilitated the electron transfer during the degradation of toluene. To further understand the catalytic mechanisms, density functional theory (DFT) calculations were employed to elucidate the interactions between toluene molecules and the Cu-modified biochar surface. These findings reveal that the strategic modification of biochar as a carrier not only enhances the dispersion and stability of active metal species but contributes to improved catalytic performance, thereby enhancing its degradation efficiency for VOCs in high-temperature conditions.
Keywords: VOCs; biochar; catalytic oxidation technology; copper.
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose. The research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Cheng K., Zhou X.Y., Wang Y., Li J.Y., Yu S.G., Liu H.J., Jiang J.S., Yi P. Analysis of emission characteristics and driving forces of air pollutants and GHG from coal-fired industrial boilers in China. J. Clean. Prod. 2023;430:139768. doi: 10.1016/j.jclepro.2023.139768. - DOI
-
- Wang Y., Arandiyan H., Scott J., Bagheri A., Dai H.X., Amal R. Recent advances in ordered meso/macroporous metal oxides for heterogeneous catalysis: A review. J. Mater. Chem. A. 2017;5:8825–8846. doi: 10.1039/C6TA10896B. - DOI
-
- Wissem B.S., Sun J., Wang W.L., Song Z.L., Zhao X.Q., Mao Y.P., Zhang Z.C. Discovering the key role of MnO2 and CeO2 particles in the Fe2O3 catalysts for enhancing the catalytic oxidation of VOC: Synergistic effect of the lattice oxygen species and surface-adsorbed oxygen. Sci. Total Environ. 2022;819:152844. doi: 10.1016/j.scitotenv.2021.152844. - DOI - PubMed
-
- Song H., Hu F.Y., Peng Y., Li K.Z., Bai S.P., Li J.H. Non-thermal plasma catalysis for chlorobenzene removal over CoMn/TiO2 and CeMn/TiO2: Synergistic effect of chemical catalysis and dielectric constant. Chem. Eng. J. 2018;347:447–454. doi: 10.1016/j.cej.2018.04.018. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

