Adipocytes Promote Cisplatin Resistance through Secreting A1BG and Regulating NAMPT/PARP1 Axis-Mediated DNA Repair in Osteosarcoma
- PMID: 40560034
- PMCID: PMC12463075
- DOI: 10.1002/advs.202502926
Adipocytes Promote Cisplatin Resistance through Secreting A1BG and Regulating NAMPT/PARP1 Axis-Mediated DNA Repair in Osteosarcoma
Abstract
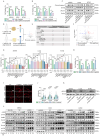
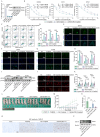
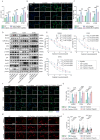
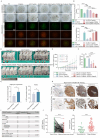
Obesity is increasingly recognized as a negative prognostic factor for cancers, including osteosarcoma. However, the mechanisms linking obesity to chemoresistance in osteosarcoma remain unclear. This study found obesity is significantly associated with poor responses to cisplatin-based chemotherapy in osteosarcoma patients. In vitro, adipocyte-conditioned medium (Adi-CM) induced cisplatin resistance, while peritumoral adipocytes and diet-induced obesity (DIO) reduce the cisplatin efficacy in vivo. Mechanistically, Adi-CM enhanced DNA repair by the PARP1/ATM pathway activation. Proteomic analysis identified A1BG, a secreted protein upregulated in adipocytes from chemoresistant patients, as a key mediator of this effect. A1BG depletion in adipocytes restored cisplatin sensitivity, whereas recombinant A1BG enhanced resistance and promoted DNA repair. Further investigation revealed a direct interaction between A1BG and NAMPT, leading to the stabilization of NAMPT and an increased NAD+ production. This enhanced PARP1 activity and subsequent DNA repair. Importantly, pharmacological inhibition of NAMPT and PARP1 using FK886 and Olaparib, respectively, reversed Adi-CM-induced cisplatin resistance and restored cisplatin sensitivity in osteosarcoma cells, DIO mouse models, and patient-derived organoids. A novel link between obesity and cisplatin resistance in osteosarcoma is established, highlighting the A1BG/NAMPT/PARP1 axis as a critical driver. Targeting this axis may represent a promising therapeutic strategy for overcoming obesity-associated chemoresistance in osteosarcoma.
Keywords: adipocytes; cisplatin resistance; dna repair; osteosarcoma.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- a) Anwar M. A., El‐Baba C., Elnaggar M. H., Elkholy Y. O., Mottawea M., Johar D., Al Shehabi T. S., Kobeissy F., Moussalem C., Massaad E., Omeis I., Darwiche N., Eid A. H., Semin. Cancer Biol. 2020, 64, 83; - PubMed
- b) Tsuda Y., Tsoi K., Parry M. C., Stevenson J. D., Fujiwara T., Sumathi V., Jeys L. M., Bone Joint J. 2020, 102‐b, 795. - PubMed
-
- a) Altaf S., Enders F., Jeavons E., Krailo M., Barkauskas D. A., Meyers P., Arndt C., Pediatr. Blood Cancer 2013, 60, 2042; - PubMed
- b) Maimaitiyiming M., Yang H., Zhou L., Zhang X., Cai Q., Wang Y., BMC Med. 2023, 21, 251; - PMC - PubMed
- c) Seke Etet P. F., Vecchio L., Nwabo Kamdje A. H., Mimche P. N., Njamnshi A. K., Adem A., Semin. Cancer Biol. 2023, 94, 50; - PubMed
- d) Hardaway A. L., Herroon M. K., Rajagurubandara E., Podgorski I., Cancer Metastasis Rev. 2014, 33, 527; - PMC - PubMed
- e) Neuhouser M. L., Aragaki A. K., Prentice R. L., Manson J. E., Chlebowski R., Carty C. L., Ochs‐Balcom H. M., Thomson C. A., Caan B. J., Tinker L. F., Urrutia R. P., Knudtson J., Anderson G. L., JAMA Oncol 2015, 1, 611; - PMC - PubMed
- f) Zhou X., Zhang J., Lv W., Zhao C., Xia Y., Wu Y., Zhang Q., J. Exp. Clin. Cancer Res. 2022, 41, 203. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
