A branched peptide targets virus and host to block influenza virus and rhinovirus entry
- PMID: 40560073
- PMCID: PMC12326976
- DOI: 10.1128/aac.00024-25
A branched peptide targets virus and host to block influenza virus and rhinovirus entry
Abstract
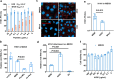
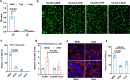
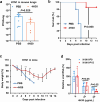
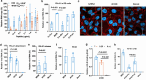
The global burden of influenza virus and rhinovirus, along with significant mortality and severe case reports, underscores the urgent need for new antivirals. Human defensins serve as the first line of defense against viruses; however, the antiviral activity of defensin peptides is often sensitive to salt, which affects their effectiveness. This study investigates a branched human-defensin peptide H30 (4H30) that can more effectively inhibit influenza virus and rhinovirus compared to the linear form of H30. Mechanistic studies reveal that 4H30 binds to influenza HA to aggregate the virus, thereby blocking viral entry. 4H30 can also cross-link H1N1 virus with cell surface glycosaminoglycans to prevent viral release. The dual-functional peptide 4H30 protects mice from the lethal challenge of the A(H1N1)pdm09 virus, demonstrating a high barrier to viral resistance after 15 viral-culture passages in the presence of 4H30. Notably, 4H30 interferes with the low-density lipoprotein receptor (LDLR) to impede the entry of minor group rhinovirus and significantly inhibits rhinovirus replication in RD cells, nasal organoids, and stem cell-derived cardiomyocytes. These findings suggest that the branched peptide 4H30, targeting both the virus and host, can more effectively inhibit influenza and rhinovirus than the linear H30, providing a new avenue for antiviral peptide development.
Keywords: antiviral peptide; human defensin peptide; influenza virus; rhinovirus; viral entry.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Integrated In Silico, In Vitro, and In Vivo Studies Reveal Mangiferin as a Promising Antiviral Agent Against H1N1/pdm2009 Influenza Virus.Viruses. 2025 Jun 21;17(7):873. doi: 10.3390/v17070873. Viruses. 2025. PMID: 40733492 Free PMC article.
-
Temporin G, an amphibian antimicrobial peptide against influenza and parainfluenza respiratory viruses: Insights into biological activity and mechanism of action.FASEB J. 2021 Feb;35(2):e21358. doi: 10.1096/fj.202001885RR. FASEB J. 2021. PMID: 33538061 Free PMC article.
-
Inhibitory Activity of Hydroxypropyl Methylcellulose on Rhinovirus and Influenza A Virus Infection of Human Nasal Epithelial Cells.Viruses. 2025 Mar 6;17(3):376. doi: 10.3390/v17030376. Viruses. 2025. PMID: 40143304 Free PMC article.
-
Physical interventions to interrupt or reduce the spread of respiratory viruses.Cochrane Database Syst Rev. 2023 Jan 30;1(1):CD006207. doi: 10.1002/14651858.CD006207.pub6. Cochrane Database Syst Rev. 2023. PMID: 36715243 Free PMC article.
-
Systematic review of influenza resistance to the neuraminidase inhibitors.BMC Infect Dis. 2011 May 19;11:134. doi: 10.1186/1471-2334-11-134. BMC Infect Dis. 2011. PMID: 21592407 Free PMC article.
References
-
- Shannon KL, Osula VO, Shaw-Saliba K, Hardick J, McBryde B, Dugas A, Hsieh Y-H, Hansoti B, Rothman RE, Emergency Department National Influenza Network Investigators . 2022. Viral co-infections are associated with increased rates of hospitalization in those with influenza. Influenza Other Respir Viruses 16:780–788. doi: 10.1111/irv.12967 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

