Design and characterization of AgVO3-HAP/GO@PCL ceramic-based scaffolds for enhanced wound healing and tissue regeneration
- PMID: 40560310
- PMCID: PMC12198066
- DOI: 10.1007/s10856-025-06907-1
Design and characterization of AgVO3-HAP/GO@PCL ceramic-based scaffolds for enhanced wound healing and tissue regeneration
Abstract
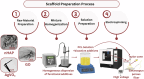
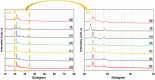
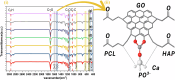
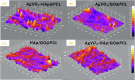
Rapid, infection-free wound healing remains a critical challenge in regenerative medicine. This study presents the fabrication and evaluation of multifunctional electrospun polycaprolactone (PCL)-based scaffolds incorporating silver vanadate (AgVO3), hydroxyapatite (HAp), and graphene oxide (GO) for advanced wound care applications. AgVO3 offers potent antibacterial properties, HAp supports osteogenic and regenerative activities and GO enhances both mechanical performance and cellular interactions. The scaffolds exhibited a highly porous nanofibrous structure, mimicking the extracellular matrix (ECM) and promoting cell attachment, migration, and nutrient exchange. Comprehensive physicochemical characterization using X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR), Raman spectroscopy, and field-emission scanning electron microscopy (FE-SEM) confirmed the successful integration of the composite. Mechanical testing revealed that GO-containing scaffolds significantly improved stiffness, with AgVO3/GO@PCL and HAp/GO@PCL achieving Young's moduli of 5.82 MPa and 4.36 MPa, respectively, which are substantially higher than that of neat PCL (1.39 MPa). In terms of flexibility, HAp/GO@PCL displayed the highest elongation at break (107.54%), indicating exceptional stretchability. The ultimate tensile strength was also enhanced in HAp@PCL (0.80 kJ/m3) and AgVO3/@PCL (0.88 kJ/m3), highlighting their capacity to resist mechanical stress during application. Contact angle measurements showed that the AgVO3-HAp/GO@PCL scaffold had the highest hydrophilicity (65.58° ± 5.97), compared to pure PCL (89.89° ± 3.70), indicating improved wettability, which is critical for fluid management and cell-material interactions at the wound interface. In vivo wound healing studies using a full-thickness rat model demonstrated that AgVO₃/GO@PCL scaffolds achieved 50% wound closure within 3 days, while AgVO₃-HAp/GO@PCL scaffolds facilitated complete re-epithelialization by day 14. Histological analysis confirmed enhanced collagen deposition and organized tissue architecture. The scaffolds also exhibited strong antibacterial activity, with large inhibition zones against S. aureus and E. coli. These findings position AgVO₃-HAp/GO@PCL scaffolds as promising candidates for next-generation wound dressings, offering a robust combination of mechanical resilience, bioactivity, antimicrobial efficacy, and moisture balance tailored for clinical wound-healing applications.
© 2025. The Author(s).
Conflict of interest statement
Compliance with ethical standards. Conflict of interest: The authors confirm that there are no conflicts of interest associated with the publication of this paper. The research was carried out objectively and without any bias. There are no financial, personal, or professional connections that could have influenced the analysis or reporting of the results. All authors have reviewed and approved the final version of the manuscript and consent to its submission.
Figures





Similar articles
-
Fabrication of biodegradable nanocomposite scaffolds with hydroxyapatite, magnetic clay, and graphene oxide for bone tissue engineering.Sci Rep. 2025 Jul 1;15(1):22235. doi: 10.1038/s41598-025-07270-5. Sci Rep. 2025. PMID: 40595079 Free PMC article.
-
In vitro characterization of 3D printed polycaprolactone/graphene oxide scaffolds impregnated with alginate and gelatin hydrogels for bone tissue engineering.J Biomater Appl. 2025 Sep;40(3):374-388. doi: 10.1177/08853282251336552. Epub 2025 Apr 25. J Biomater Appl. 2025. PMID: 40278887
-
Fabrication and Characterization of 3D Printed Polycaprolactone/Baghdadite/Zinc Oxide Nanocomposite Scaffolds for Bone Tissue Engineering.Biopolymers. 2025 Sep;116(5):e70041. doi: 10.1002/bip.70041. Biopolymers. 2025. PMID: 40736156
-
Skin Regeneration and Wound Healing by Plant Protein-Based Electrospun Fiber Scaffolds and Patches for Tissue Engineering Applications.Macromol Rapid Commun. 2025 Jul;46(13):e2500196. doi: 10.1002/marc.202500196. Epub 2025 May 29. Macromol Rapid Commun. 2025. PMID: 40441898 Review.
-
Dressings and topical agents for treating venous leg ulcers.Cochrane Database Syst Rev. 2018 Jun 15;6(6):CD012583. doi: 10.1002/14651858.CD012583.pub2. Cochrane Database Syst Rev. 2018. PMID: 29906322 Free PMC article.
References
-
- Uberoi A, McCready-Vangi A, Grice EA. The wound microbiota: microbial mechanisms of impaired wound healing and infection. Nat Rev Microbiol. 2024;22:507–21. - PubMed
-
- Sanjarnia P, Picchio ML, Polegre Solis AN, Schuhladen K, Fliss PM, Politakos N, et al. Bringing innovative wound care polymer materials to the market: challenges, developments, and new trends. Adv Drug Deliv Rev. 2024;207:115217. - PubMed
-
- Srivastava GK, Martinez-Rodriguez S, Md Fadilah NI, Looi Qi Hao D, Markey G, Shukla P, et al. Progress in Wound-Healing Products Based on Natural Compounds, Stem Cells, and MicroRNA-Based Biopolymers in the European, USA, and Asian Markets: Opportunities, Barriers, and Regulatory Issues. Polymers; 2024. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

