FDA's insights: implementing new strategies for evaluating drug-induced QTc prolongation
- PMID: 40560402
- PMCID: PMC12198268
- DOI: 10.1007/s10928-025-09985-4
FDA's insights: implementing new strategies for evaluating drug-induced QTc prolongation
Abstract
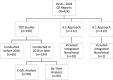
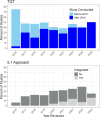
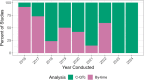
The questions and answers (Q&A) document for ICH E14/S7B provides the following advancements for QTc assessment: concentration-QTc modeling (C-QTc) as the primary analysis, accepting alternative approaches (Q&A 5.1 and 6.1) to thorough QT (TQT) studies, and incorporating an integrated nonclinical risk assessment as supporting evidence. Based on QT study reports reviewed by the FDA between 2016 and 2024, changes to the E14 guideline have resulted in a 34% decrease in the proportion of TQT studies, while the use of C-QTc analysis as the primary analysis has significantly increased. Studies using C-QTc instead of by-time analysis as the primary analysis reduced median sample sizes by 67%, 42%, and 35% for parallel, nested crossover, and crossover studies, respectively. The white paper C-QTc model was used for 60% of drugs that prolonged the QTc interval. From 2020 to 2024, reviews incorporating an integrated nonclinical risk assessment have also increased. The advancements in QTc assessments have streamlined QTc assessment and made clinical trials less resource-intensive. As the advancements continue to evolve the drug safety evaluation is likely to become even more adaptive and enable more precise and targeted QTc assessment.
Keywords: Electrocardiogram; ICH E14 guideline; QTc interval prolongation; Thorough QT study.
© 2025. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Do Studies Evaluating QT/QTc Interval Prolongation with Dietary Supplements Meet FDA Standards: A Systematic Review.J Diet Suppl. 2017 Jul 4;14(4):467-477. doi: 10.1080/19390211.2016.1253633. Epub 2016 Dec 12. J Diet Suppl. 2017. PMID: 27937000
-
Results from a Joined Prospective Study to Evaluate the Sensitivity of the In Vivo Dog QT Assay in Line with the ICH E14/S7B Q&A Best Practices.Clin Pharmacol Ther. 2024 Jul;116(1):106-116. doi: 10.1002/cpt.3283. Epub 2024 May 6. Clin Pharmacol Ther. 2024. PMID: 38709223
-
Lessons learned from QT prolongation risk assessment for antibody-drug conjugates in oncology.J Pharmacokinet Pharmacodyn. 2025 Jul 28;52(4):44. doi: 10.1007/s10928-025-09988-1. J Pharmacokinet Pharmacodyn. 2025. PMID: 40721543 Review.
-
A comprehensive review of 20 years of progress in nonclinical QT evaluation and proarrhythmic assessment.J Pharmacokinet Pharmacodyn. 2025 May 16;52(3):32. doi: 10.1007/s10928-025-09979-2. J Pharmacokinet Pharmacodyn. 2025. PMID: 40379846 Review.
References
-
- ICH Expert Working Group (2005) Guidance for Industry: E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs. https://www.fda.gov/media/71372/download. Accessed 01/01/2025
-
- Garnett CE, Beasley N, Bhattaram VA, Jadhav PR, Madabushi R, Stockbridge N, Tornoe CW, Wang Y, Zhu H, Gobburu JV (2008) Concentration-QT relationships play a key role in the evaluation of proarrhythmic risk during regulatory review. J Clin Pharmacol 48(1):13–18. 10.1177/0091270007307881 - PubMed
-
- Vargas HM, Bass AS, Breidenbach A, Feldman HS, Gintant GA, Harmer AR, Heath B, Hoffmann P, Lagrutta A, Leishman D, McMahon N, Mittelstadt S, Polonchuk L, Pugsley MK, Salata JJ, Valentin JP (2008) Scientific review and recommendations on preclinical cardiovascular safety evaluation of biologics. J Pharmacol Toxicol Methods 58(2):72–76. 10.1016/j.vascn.2008.04.001 - PubMed
-
- Rodriguez I, Erdman A, Padhi D, Garnett CE, Zhao H, Targum SL, Balakrishnan S, Strnadova C, Viner N, Geiger MJ, Newton-Cheh C, Litwin J, Pugsley MK, Sager PT, Krucoff MW, Finkle JK (2010) Electrocardiographic assessment for therapeutic proteins–scientific discussion. Am Heart J 160(4):627–634. 10.1016/j.ahj.2010.07.001 - PubMed
-
- ICH Expert Working Group (2022) Guidance for Industry: E14 and S7B Clinical and Nonclinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential--Questions and Answers https://www.fda.gov/media/161198/download. Accessed 01/01/2025
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

