Tumor-promoting function of paternally expressed gene 10 and its immunogenicity in inducing anti-tumor helper T cells in head and neck squamous cell carcinoma
- PMID: 40560436
- PMCID: PMC12198103
- DOI: 10.1007/s00262-025-04110-3
Tumor-promoting function of paternally expressed gene 10 and its immunogenicity in inducing anti-tumor helper T cells in head and neck squamous cell carcinoma
Abstract
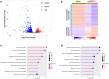
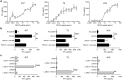
Paternally expressed gene 10 (PEG10) is expressed primarily in the placenta; its expression is extremely low or absent in normal tissues but up-regulated in various cancers, indicating that PEG10 is a potential target for cancer immunotherapy. However, the expression and role of PEG10 in head and neck squamous cell carcinoma (HNSCC) and the immunogenicity of possible PEG10-derived T-cell epitopes remain unclear. In the present study, we show that PEG10 is expressed in HNSCC, and its high expression is associated with poor patient survival. Suppression of PEG10 expression attenuated the proliferation, migration, and invasion of HNSCC cells and altered their gene expression profiles. We also identified a PEG10-derived peptide epitope (PEG10216-232) capable of eliciting antigen-specific and promiscuous human leukocyte antigen (HLA)-DR-restricted helper T lymphocyte (HTL) responses. Notably, PEG10-specific HTLs exerted direct cytotoxicity against PEG10-positive HNSCC cells in an HLA-DR-restricted manner. Moreover, precursor T cells that react to PEG10216-232 peptide were detected in HNSCC patients. These results indicate that PEG10 plays an important role in HNSCC tumorigenesis and qualifies as an immunotherapeutic target against HNSCC. The helper epitope peptide of PEG10 could effectively stimulate antigen-specific HTLs and induce anti-tumor responses against PEG10-positive cancers, including HNSCC.
Keywords: Epitope; Head and neck squamous cell carcinoma; Helper T lymphocytes; Paternally expressed gene 10; Peptide vaccine; Placenta.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no competing interests. Ethical approval: This study was performed in compliance with the principles of the Declaration of Helsinki and subsequent amendments, and the study protocol was approved by the Institutional Review Board of the Asahikawa Medical University (#15005 and #16217). All animal experiments were approved by the Institutional Animal Care and Use Committee of Asahikawa Medical University (R6-035). Informed consent: As this was a retrospective study using existing specimens and clinical information, the Institutional Review Board approved a waiver of the standard informed consent process for IHC analyses. Instead, an opt-out approach was implemented through the Asahikawa Medical University website that ensured patients retained the right to decline participation in the study. All patients from whom PBMCs were collected received a written explanation of the study and provided written informed consent before participation.
Figures




References
-
- Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, Worden F, Saba NF, Iglesias Docampo LC, Haddad R, Rordorf T, Kiyota N, Tahara M, Monga M, Lynch M, Geese WJ, Kopit J, Shaw JW, Gillison ML (2016) Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 375:1856–1867. 10.1056/NEJMoa1602252 - DOI - PMC - PubMed
-
- Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA, Restifo NP, Allison JP (2010) Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med 207:637–650. 10.1084/jem.20091918 - DOI - PMC - PubMed
-
- Oh DY, Kwek SS, Raju SS, Li T, McCarthy E, Chow E, Aran D, Ilano A, Pai CS, Rancan C, Allaire K, Burra A, Sun Y, Spitzer MH, Mangul S, Porten S, Meng MV, Friedlander TW, Ye CJ, Fong L (2020) Intratumoral CD4(+) T cells mediate anti-tumor cytotoxicity in human bladder cancer. Cell 181(1612–1625):e1613. 10.1016/j.cell.2020.05.017 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

