Guselkumab for Moderate to Severe Scalp Psoriasis Across All Skin Tones: Cohort B of the VISIBLE Randomized Clinical Trial
- PMID: 40560554
- PMCID: PMC12199185
- DOI: 10.1001/jamadermatol.2025.1849
Guselkumab for Moderate to Severe Scalp Psoriasis Across All Skin Tones: Cohort B of the VISIBLE Randomized Clinical Trial
Abstract
Importance: Varying hair textures and hair care practices contribute to nuances in clinical presentation and management of scalp psoriasis across diverse patient populations. Cohort B of the VISIBLE trial enrolled participants with moderate to severe scalp psoriasis and skin of color, across the skin-tone spectrum.
Objective: To evaluate efficacy, quality of life, and adverse event outcomes of guselkumab, 100 mg, among participants with moderate to severe scalp psoriasis and skin of color over 48 weeks.
Design, setting, and participants: This ongoing phase 3b, randomized clinical trial at 45 sites in the US and Canada enrolled adults with skin of color and moderate to severe scalp psoriasis (scalp surface area [SSA] ≥30%; Psoriasis Scalp Severity Index [PSSI] ≥12; scalp-specific Investigator's Global Assessment [ss-IGA] score ≥3; ≥1 nonscalp plaque). Data were collected from September 2022 to June 2024.
Interventions: Randomized participants (3:1) received guselkumab, 100 mg, at weeks 0, 4, and every 8 weeks, or placebo at weeks 0, 4, and 12, then guselkumab at weeks 16, 20, and every 8 weeks.
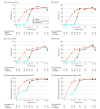
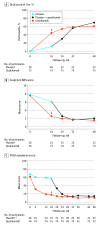
Main outcomes and measures: Coprimary end points were ss-IGA score of 0 or 1 (ss-IGA 0/1) and 90% or greater improvement in PSSI (PSSI 90) at week 16 (guselkumab vs placebo). Major secondary end points included ss-IGA 0 (complete scalp clearance), PSSI 100, percentage changes from baseline in PSSI and SSA, changes from baseline in Dermatology Life Quality Index (DLQI) and Psoriasis Symptoms and Signs Diary (PSSD) symptoms score, and 4-point or greater reduction in Scalp Itch Numeric Rating Scale (NRS) score.
Results: Of 108 participants (81 randomized to guselkumab; 27 randomized to placebo), 100 (92.6%) completed 48 weeks of treatment. The mean (SD) age overall was 42.5 (13.6) years, and 58 participants (56.9%) were male. At the week 16 primary end point, in the guselkumab (n = 76) vs placebo (n = 26) groups, respectively, response rates were as follows: ss-IGA 0/1, 68.4% (n = 52) vs 11.5% (n = 3) (P < .001); PSSI 90, 65.8% (n = 50) vs 3.8% (n = 1) (P < .001); ss-IGA 0, 57.9% (n = 44) vs 3.8% (n = 1) (P < .001); PSSI 100, 59.2% (n = 45) vs 3.8% (n = 1) (P < .001); 4-point or greater reduction in Scalp Itch NRS score (in those with a baseline score of at least 4), 69.4% (n = 50 of 72) vs 24.0% (n = 6 of 25) (P < .001). Guselkumab efficacy increased and was maintained through week 48, when guselkumab-randomized participants achieved mean (SD) percentage improvements in PSSI and SSA of 94.6% (12.2%) and 94.8% (16.2%), respectively, and 51 (67.1%) achieved ss-IGA 0. DLQI and PSSD symptoms score least-squares mean changes were -9.7 (95% CI, -11.1 to -8.2) vs -2.2 (95% CI, -4.8 to 0.4) (P < .001) and -44.8 (95% CI, -50.6 to -39.1) vs -8.3 (95% CI, -18.4 to 1.9) (P < .001), respectively, with sustained improvements through week 48. Through week 16, infections were the most common adverse events in the guselkumab (n = 12; 14.8%) and placebo (n = 1; 3.7%) groups.
Conclusions and relevance: In this randomized clinical trial, after 3 doses of guselkumab, most participants achieved significant scalp clearance and clinically meaningful quality-of-life improvements; improvements increased and were maintained through week 48.
Trial registration: ClinicalTrials.gov Identifier: NCT05272150.
Conflict of interest statement
Figures

References
-
- Chang CC, Gangaram HB, Hussein SH. Malaysian Psoriasis Registry—preliminary report of a pilot study using a newly revised registry form. Med J Malaysia. 2008;63 Suppl C:68-71. - PubMed
-
- Crowley J. Scalp psoriasis: an overview of the disease and available therapies. J Drugs Dermatol. 2010;9(8):912-918. - PubMed
-
- Chatrath S, Bradley L, Kentosh J. Dermatologic conditions in skin of color compared to white patients: similarities, differences, and special considerations. Arch Dermatol Res. 2023;315(5):1089-1097. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

