Trichomonas vaginalis extracellular vesicles suppress IFNε-mediated responses driven by its intracellular bacterial symbiont Mycoplasma hominis
- PMID: 40560611
- PMCID: PMC12232435
- DOI: 10.1073/pnas.2508297122
Trichomonas vaginalis extracellular vesicles suppress IFNε-mediated responses driven by its intracellular bacterial symbiont Mycoplasma hominis
Abstract
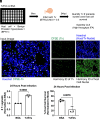
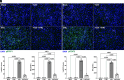
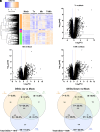
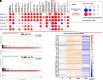
Trichomonas vaginalis is a common, extracellular, sexually transmitted parasite which is often found in symbiosis with the intracellular bacterium Mycoplasma hominis (Mh), an opportunistic pathogen of the female reproductive tract. How this symbiosis affects infection outcomes and the host cell innate immune response is poorly understood. Here, we show that infection with T. vaginalis in symbiosis with M. hominis or M. hominis alone triggers a noncanonical type I interferon, interferon-epsilon (IFNε), but infection with T. vaginalis alone does not. We also demonstrate that extracellular vesicles (TvEVs) produced by the parasite downregulate host cell IFNε, counteracting this symbiont-driven response and elevating infection. We further demonstrate that IFNε, a hormonally regulated cytokine produced in the human reproductive system, is protective against T. vaginalis cytoadherence and cytolysis of host cells. These studies provide insight into how a parasite and its bacterial symbiont work in concert to regulate host cell innate immune responses to drive infection.
Keywords: Mycoplasma hominis; Trichomonas vaginalis; extracellular vesicles; innate immunity; interferon.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

Similar articles
-
Trichomonas vaginalis and Mycoplasma hominis: new tales of two old friends.Parasitology. 2019 Aug;146(9):1150-1155. doi: 10.1017/S0031182018002135. Epub 2019 Jan 8. Parasitology. 2019. PMID: 30616707 Review.
-
Association of Trichomonas vaginalis with its symbiont Mycoplasma hominis synergistically upregulates the in vitro proinflammatory response of human monocytes.Sex Transm Infect. 2013 Sep;89(6):449-54. doi: 10.1136/sextrans-2012-051006. Epub 2013 Apr 30. Sex Transm Infect. 2013. PMID: 23633668
-
Mycoplasma hominis shows strain-dependent increase in resistance to selected antibiotics after symbiosis with Trichomonas vaginalis.J Glob Antimicrob Resist. 2018 Sep;14:169-175. doi: 10.1016/j.jgar.2018.04.003. Epub 2018 Apr 13. J Glob Antimicrob Resist. 2018. PMID: 29660412
-
Mycoplasma hominis and Trichomonas vaginalis symbiosis: multiplicity of infection and transmissibility of M. hominis to human cells.Arch Microbiol. 2001 Jan;175(1):70-4. doi: 10.1007/s002030000240. Arch Microbiol. 2001. PMID: 11271423
-
Mycoplasma hominis and Trichomonas vaginalis: a unique case of symbiotic relationship between two obligate human parasites.Front Biosci. 2006 Sep 1;11:2028-34. doi: 10.2741/1944. Front Biosci. 2006. PMID: 16720288 Review.
References
-
- Dalby J., Stoner B. P., Sexually transmitted infections: Updates from the 2021 CDC guidelines. Am. Fam. Physician 105, 514–520 (2022). - PubMed
-
- Yang S., et al. , Trichomonas vaginalis infection-associated risk of cervical cancer: A meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 228, 166–173 (2018). - PubMed
MeSH terms
Grants and funding
- T32 AI007323/AI/NIAID NIH HHS/United States
- F32 AI186416/AI/NIAID NIH HHS/United States
- R01 AI103182/AI/NIAID NIH HHS/United States
- R01AI103182/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- T32AI007323/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
LinkOut - more resources
Full Text Sources

