Distinct adaptation and epidemiological success of different genotypes within Salmonella enterica serovar Dublin
- PMID: 40560760
- PMCID: PMC12194135
- DOI: 10.7554/eLife.102253
Distinct adaptation and epidemiological success of different genotypes within Salmonella enterica serovar Dublin
Abstract
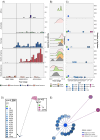
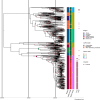
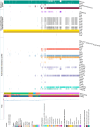
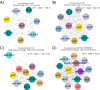
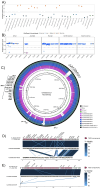
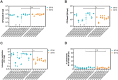
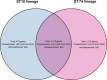
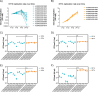
Salmonella Dublin is a host-adapted, invasive nontyphoidal Salmonella (iNTS) serovar that causes bloodstream infections in humans and demonstrates increasing prevalence of antimicrobial resistance (AMR). Using a global dataset of 1303 genomes, coupled with in vitro assays, we examined the evolutionary, resistance, and virulence characteristics of S. Dublin. Our analysis revealed strong geographical associations between AMR profiles and plasmid types, with highly resistant isolates confined predominantly to North America, linked to IncC plasmids co-encoding AMR and heavy metal resistance. By contrast, Australian isolates were largely antimicrobial-susceptible, reflecting differing AMR pressures. We identified two phylogenetically distinct Australian lineages, ST10 and ST74, with a small number of ST10 isolates harbouring a novel hybrid plasmid encoding both AMR and mercuric resistance. Whereas the ST10 lineage remains globally dominant, the ST74 lineage was less prevalent. ST74 exhibited unique genomic features including a larger pan genome compared to ST10 and the absence of key virulence loci, including Salmonella pathogenicity island (SPI)-19 which encodes a type VI secretion system (T6SS). Despite these genomic differences, the ST74 lineage displayed enhanced intracellular replication in human macrophages and induced less pro-inflammatory responses compared with ST10, suggesting alternative virulence strategies that may support systemic dissemination of ST74. The Vi antigen was absent in all ST10 and ST74 genomes, highlighting challenges for serotyping and vaccine development, and has implications for current diagnostic and control strategies for S. Dublin infections. Collectively, this study represents the most comprehensive investigation of S. Dublin to date and, importantly, has revealed distinct adaptations of two genotypes within the same serovar, leading to different epidemiological success. The regional emergence and evolution of distinct S. Dublin lineages highlight the need to understand the divergence of intra-serovar virulence mechanisms which may impact the development of effective control measures against this important global pathogen.
Keywords: Salmonella Dublin; epidemiology; host immune response; immunology; infectious disease; inflammation; invasive nontyphoidal Salmonella; microbiology.
Plain language summary
Salmonella bacteria often live harmlessly in animals but can make people sick. For example, milk or meat from cows infected with a type called Salmonella Dublin can cause serious blood infections. Over time, many Salmonella strains have become harder to treat because they carry genes that let them survive antibiotic treatment. These survival genes often sit on small DNA circles called plasmids, which can move between bacteria. By studying the DNA from Salmonella Dublin samples from around the world, scientists can learn how different groups of this bacterium have evolved to resist drugs and cause infections in humans. Understanding these changes may help explain why some types of Salmonella Dublin spread more easily to humans and may point to better ways to detect and control infections. Sia et al. identified two main lineages of Salmonella Dublin: the widespread ST10 group and a less common ST74 group. The experiments compared DNA from more than 1300 strains of Salmonella Dublin from 13 countries on five continents. The ST10 strains from North America often had genes that made them resistant to antibiotics and heavy metals. Resistance to heavy metals may make it easier for bacteria to develop resistance to drugs. Most Australian strains of ST10 remained drug sensitive. ST74 strains lack a key secretion system gene set but have more genes overall than ST10. In laboratory tests using human immune cells, ST74 bacteria multiplied up to eight times more over 24 hours and triggered weaker immune responses in the cells than ST10. Both groups of bacteria lacked the Vi capsule gene, which some vaccines against bacterial infections have targeted. The experiments may also help explain why some strains of the bacteria cause more disease and point to new ways to prevent or treat infections. Knowing that ST74 does not trigger a strong immune response may help scientists develop new therapies to strengthen the immune response and prevent the bacteria from multiplying and spreading. Vaccines targeting the Vi capsule may not be effective against Salmonella Dublin, because most strains do not have it. Instead, scientists may want to use the data to look for alternate targets. More research is needed to support the study's findings.
© 2024, Sia et al.
Conflict of interest statement
CS, RA, MV, PA, SB, BH, DW, JP, DI No competing interests declared
Figures







Update of
- doi: 10.1101/2024.07.30.605691
- doi: 10.7554/eLife.102253.1
- doi: 10.7554/eLife.102253.2
- doi: 10.7554/eLife.102253.3
References
-
- Achtman M, Wain J, Weill FX, Nair S, Zhou Z, Sangal V, Krauland MG, Hale JL, Harbottle H, Uesbeck A, Dougan G, Harrison LH, Brisse S, S. Enterica MLST Study Group Multilocus sequence typing as a replacement for serotyping in Salmonella enterica. PLOS Pathogens. 2012;8:e1002776. doi: 10.1371/journal.ppat.1002776. - DOI - PMC - PubMed
-
- Amaya FA, Blondel CJ, Barros-Infante MF, Rivera D, Moreno-Switt AI, Santiviago CA, Pezoa D. Identification of Type VI secretion systems effector proteins that contribute to interbacterial competition in Salmonella Dublin. Frontiers in Microbiology. 2022;13:811932. doi: 10.3389/fmicb.2022.811932. - DOI - PMC - PubMed
-
- Ayres DL, Darling A, Zwickl DJ, Beerli P, Holder MT, Lewis PO, Huelsenbeck JP, Ronquist F, Swofford DL, Cummings MP, Rambaut A, Suchard MA. BEAGLE: an application programming interface and high-performance computing library for statistical phylogenetics. Systematic Biology. 2012;61:170–173. doi: 10.1093/sysbio/syr100. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

