Unlocking a Nano-Aluminum Oxide Lewis Acid Layer as the Electrocatalyst for Hydrogenation of Thiophene and Other Arenes
- PMID: 40560765
- PMCID: PMC12257536
- DOI: 10.1021/jacs.5c06034
Unlocking a Nano-Aluminum Oxide Lewis Acid Layer as the Electrocatalyst for Hydrogenation of Thiophene and Other Arenes
Abstract
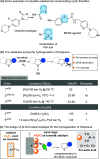
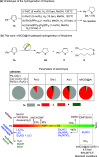
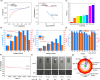
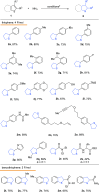
Owing to the ready formation of an insulating oxide layer, there is an intrinsic obstacle to the use of aluminum material as the cathode in the electrocatalytic reaction. In this report, a new Al-based material with a surface aluminum oxide/chloride nanolayer (nAlClO@Al) is prepared. Via the quantum tunneling effect, this material realizes conductivity through the nano-Al-O layer. This material exhibits unusual inertness toward the hydrogen evolution reaction, leaving a wide reduction window for the substrate. Primed with the native Lewis acidity of the Al-Cl structure, this material achieves the electrocatalytic hydrogenation of thiophenes with up to >95% conversion, >95% selectivity, and 90% Faraday efficiency. This catalysis is applicable to heteroarene and benzene rings. In particular, the chemoselectivity is controlled by the nAlClO@Al catalyst instead of the substrate, which is complementary to electrochemical Birch chemistry.
Figures



References
-
- Gu X., Li X., Xie J., Zhou Q.. Recent Progress in Homogeneous Catalytic Hydrogenation of Esters. Acta Chim. Sinica. 2019;77:598–612. doi: 10.6023/A19050166. - DOI
LinkOut - more resources
Full Text Sources

