Sugar type and route of delivery influence insulin and glucose-dependent insulinotropic polypeptide responses in rats
- PMID: 40560801
- PMCID: PMC12320374
- DOI: 10.1152/ajpendo.00460.2024
Sugar type and route of delivery influence insulin and glucose-dependent insulinotropic polypeptide responses in rats
Abstract
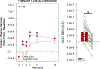
To help resolve the characteristics of orally stimulated endocrine responses to sugar, we developed a novel rat preparation with surgically implanted intraoral (IO) and intragastric (IG) cannulas for stimulus delivery, along with jugular vein catheters for blood sampling, and tested the effects of 1-min and 10-min IO versus IG infusions (1 mL/min) of 1.0 M glucose on plasma levels of insulin, glucose-dependent insulinotropic polypeptide (GIP), and glucose. Oral glucose delivery (1 min and 10 mins) caused a greater (P ≤ 0.05) early rise (1 min) in insulin levels than gastric glucose delivery, also reflected in the 3-min area under the curve (AUC). The 10-min, but not the 1-min, IO glucose infusion also caused a greater (P ≤ 0.05) increase in GIP levels than the IG infusions, as evidenced by the 3-min AUC. Oral delivery of 1.0 M fructose produced marginally (but significantly) higher insulin and GIP levels than gastric fructose delivery, although the difference appeared much smaller than that observed for isomolar glucose, suggesting some degree of chemospecificity and the involvement of a taste type 1 receptor-independent mechanism. Our triple cannulation/catheterization rat preparation is well suited to assess endocrine responses to oral stimulation. By comparing the effects of stimulus infusion into the oral cavity (oral + postoral stimulation) with the stimulus infusion directly into the stomach (only postoral stimulation), we confirmed the primacy of glucose to orally trigger an increase in circulating insulin while controlling for changes in plasma glucose. This approach offers promise for reliably characterizing orally stimulated endocrine responses in rats and potentially in other animal models as well.NEW & NOTEWORTHY We describe an innovative preparation that can effectively characterize orally stimulated endocrine responses during ongoing ingestion in rats. We found that glucose orally triggered an early rise in not only plasma insulin-which in some circumstances was present even as glycemia increased-but also glucose-dependent insulinotropic polypeptide, albeit more weakly. These endocrine responses to orally delivered fructose were weak or nonexistent, confirming the primacy of glucose as the key monosaccharide stimulus.
Keywords: GIP; cephalic phase insulin release; glucose; glucose sensing; metabolism.
Figures



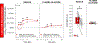
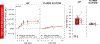

Similar articles
-
Glucose-Dependent Insulinotropic Polypeptide Is Involved in Postprandial Regulation of Splanchnic Blood Supply.Diabetes. 2025 Aug 1;74(8):1355-1366. doi: 10.2337/db25-0149. Diabetes. 2025. PMID: 40445944 Clinical Trial.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Hybrid closed-loop systems for managing blood glucose levels in type 1 diabetes: a systematic review and economic modelling.Health Technol Assess. 2024 Dec;28(80):1-190. doi: 10.3310/JYPL3536. Health Technol Assess. 2024. PMID: 39673446 Free PMC article.
-
Comparison of self-administered survey questionnaire responses collected using mobile apps versus other methods.Cochrane Database Syst Rev. 2015 Jul 27;2015(7):MR000042. doi: 10.1002/14651858.MR000042.pub2. Cochrane Database Syst Rev. 2015. PMID: 26212714 Free PMC article.
-
Preexisting Diabetes and Pregnancy: An Endocrine Society and European Society of Endocrinology Joint Clinical Practice Guideline.Eur J Endocrinol. 2025 Jun 30;193(1):G1-G48. doi: 10.1093/ejendo/lvaf116. Eur J Endocrinol. 2025. PMID: 40652450
References
-
- Spector AC. Linking gustatory neurobiology to behavior in vertebrates. Neurosci Biobehav Rev 24: 391–416, 2000. - PubMed
-
- Spector AC. The functional organization of the peripheral gustatory system: lessons from behavior. In: Progress in Psychobiology and Physiological Psychology, edited by Fluharty SJ and Grill HJ. San Diego: Academic Press, 2003.
-
- Powley TL. The ventromedial hypothalamic syndrome, satiety, and a cephalic phase hypothesis. Psychol Rev 84: 89–126, 1977. - PubMed
-
- Powley TL, Berthoud HR. Diet and cephalic phase insulin responses. Am J Clin Nutr 42: 991–1002, 1985. - PubMed
-
- Teff K Nutritional implications of the cephalic-phase reflexes: endocrine responses. Appetite 34: 206–213, 2000. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

