Economic assessment of potential changes to essential medicines for diabetes in Uganda
- PMID: 40560893
- PMCID: PMC12193067
- DOI: 10.1371/journal.pone.0326806
Economic assessment of potential changes to essential medicines for diabetes in Uganda
Abstract
Background: The World Health Organization suggests selecting essential medicines based on availability, accessibility, affordability, cost-effectiveness, and safety and efficacy. We examined Oral Hypoglycemic Agents (OHAs) for type 2 diabetes mellitus in the Essential Medicines and Health Supplies List for Uganda (EMHSLU), assessed all criteria, and proposed alternative medicines to improve cost-savings and health outcomes.
Methods: We conducted a literature review followed by budget impact analysis (BIA) to evaluate potential cost-savings from refining EMHSLU. In our literature review, we identified metrics for measuring availability, accessibility, and affordability of medicines and evaluated evidence on cost-effectiveness, safety, and efficacy of OHAs. According to the country context and available data, we used applicable methods to analyze five classes of OHAs available in Uganda. Since the government covers essential medicines in the public sector based on BIA, we assessed affordability for the government by examining potential savings in two scenarios (#1: shifts within the EMHSLU; #2: use of non-EMHSLU drugs).
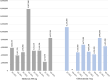
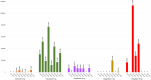
Results: OHAs added to the EMHSLU 2023 are less available and accessible compared to OHAs in EMHSLU 2016. BIA scenario 1 revealed that the complete replacement of glimepiride 2 mg and gliclazide 80 mg with glibenclamide 5 mg could save the government up to USD 2.65 million per year. Additionally, scenario 2 showed that full replacement of dapagliflozin 10 mg and glimepiride 4 mg instead of their lower doses can save up to United States Dollar (USD) 230,000 and USD 600,000 per year, respectively. A review and adaptation of a published cost-effectiveness analysis highlights that sitagliptin is a cost-effective OHA in Uganda with an estimated Incremental Cost Effectiveness Ratio of $6,132/Quality-Adjusted LifeYear.
Conclusion: The findings suggest several potential type 2 diabetes oral medication substitutions which would reduce costs.
Copyright: © 2025 Bonyani et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- World Health Organization. Selection of essential medicines at country level: using the WHO model list of essential medicines to update a national essential medicines list [Internet]. Geneva: World Health Organization; 2020. [cited 2024 May 27]. Available from: https://iris.who.int/handle/10665/330898
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical