Computer-aided discovery of dual-target compounds for Alzheimer's from ayurvedic medicinal plants
- PMID: 40560959
- PMCID: PMC12193798
- DOI: 10.1371/journal.pone.0325441
Computer-aided discovery of dual-target compounds for Alzheimer's from ayurvedic medicinal plants
Abstract
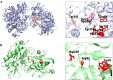
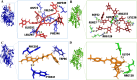
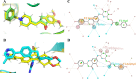
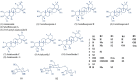
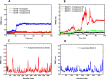
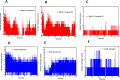
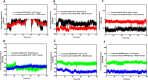
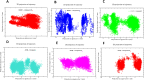
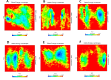
Alzheimer's disease (AD) is a neurodegenerative disorder characterized by cognitive decline, driven by the accumulation of amyloid-beta plaques and neurofibrillary tangles. It involves the dysfunction of key enzymes such as Acetylcholinesterase (AChE) and β-secretase (BACE1), making them critical targets for therapeutic intervention. In this study we investigated an in-house library of 820 secondary metabolites obtained from Ayurvedic plants against AChE and BACE1 with the aim to discover novel leads for AD. Virtual screening resulted in 15 ligands, mostly belonging to the ursane-type or dammarene-type triterpene saponins of Centella asiatica, reestablishing the potency of this plant in drug discovery against AD. The binding affinities were further verified by molecular dynamics (MD) simulation trajectories, including root mean square fluctuations (RMSF), root mean square deviation (RMSD), hydrogen bonding analysis, Coulomb interaction calculation, Lennard-Jones interactions, and the total interaction energy. Moreover, extensive Principal Component Analysis (PCA) and Gibbs free energy landscape were performed. Our results demonstrated three compounds, namely (S)-eriodictyol 7-O-(6-β-O-trans-p-coumaroyl)-β-d-glucopyranoside, sitoindoside-X and 1,5-di-o-caffeoyl quinic acid as more effective in treating AD due to their comparable drug-like properties. Drug-likeness, structural chemistry, pharmacophore, and ADMET (Absorption, Distribution, Metabolism, Excretion, and Toxicity) analysis support their potential for future drug development. To establish the effectiveness of these lead compounds against AD, additional experimental testing should be performed.
Copyright: © 2025 Shah et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Novel Thiazole-Based Compounds as Potential Beta-Site Amyloid Precursor Protein Cleaving Enzyme 1 Inhibitors for Alzheimer's Disease.Clin Lab. 2025 Aug 1;71(8). doi: 10.7754/Clin.Lab.2025.241234. Clin Lab. 2025. PMID: 40779485
-
ML-based prediction to experimental validation: Development of dihydroquinazoline based multi-potent ligands as anti-Alzheimer's agents.Comput Biol Med. 2025 Sep;196(Pt A):110762. doi: 10.1016/j.compbiomed.2025.110762. Epub 2025 Jul 14. Comput Biol Med. 2025. PMID: 40664126
-
DFT-Based Elucidation and Evaluation of Selenium-Modified Tacrine Derivatives: Theoretical and Physicochemical Insights for Alzheimer's Disease Therapy.Molecules. 2025 Jun 11;30(12):2553. doi: 10.3390/molecules30122553. Molecules. 2025. PMID: 40572521 Free PMC article.
-
Interlinking diabetes and Alzheimer's disease: A pathway through medicinal plant-based treatments.J Ethnopharmacol. 2025 Jul 24;351:120092. doi: 10.1016/j.jep.2025.120092. Epub 2025 Jun 6. J Ethnopharmacol. 2025. PMID: 40484255 Review.
-
Withdrawal or continuation of cholinesterase inhibitors or memantine or both, in people with dementia.Cochrane Database Syst Rev. 2021 Feb 3;2(2):CD009081. doi: 10.1002/14651858.CD009081.pub2. Cochrane Database Syst Rev. 2021. PMID: 35608903 Free PMC article.
References
-
- Jannat S, Balupuri A, Ali MY, Hong SS, Choi CW, Choi Y-H, et al. Inhibition of β-site amyloid precursor protein cleaving enzyme 1 and cholinesterases by pterosins via a specific structure-activity relationship with a strong BBB permeability. Exp Mol Med. 2019;51(2):1–18. doi: 10.1038/s12276-019-0205-7 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

