Core-shell microcapsules compatible with routine injection enable prime/boost immunization against malaria with a single shot
- PMID: 40560998
- PMCID: PMC7618038
- DOI: 10.1126/scitranslmed.adw2256
Core-shell microcapsules compatible with routine injection enable prime/boost immunization against malaria with a single shot
Abstract
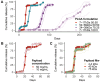
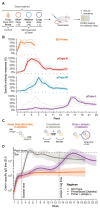
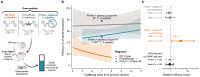
Inadequate booster uptake threatens the success of immunization campaigns as seen with the recently rolled-out R21 malaria vaccine. The ability to administer both prime and boost immunizations with a single injection would therefore save lives and alleviate health care burdens. We present a platform for delayed delivery of the booster dose that is scalable with existing technology, easily injectable, and protective against malaria in vivo. Using chip-based microfluidics, we encapsulated the R21 malaria vaccine in polymer microcapsules that release their content weeks to months postinjection. Coinjecting microcapsules with the priming dose of the R21 vaccine elicited strong antibody responses in a mouse model and provided 85% of the protection of a standard prime/boost schedule. If confirmed in humans, these results would pave the way for rapid deployment of single-shot prime/boost vaccination, an urgently needed global health intervention.
Conflict of interest statement
R.G., A.M., E.S., and A.V.S.H. are named inventors on patent family WO 2023/194745, filed by Oxford University Innovation Ltd, describing the use of a stepped design microfluidic chip for preparation of core-shell microcapsules. A.V.S.H. is named as a co-inventor on patents related to the R21 malaria vaccine.
Figures



References
-
- Vaccination and Immunization Statistics - UNICEF DATA. (available at https://data.unicef.org/topic/child-health/immunization/)
-
- WHO Immunization Data portal. (available at https://immunizationdata.who.int/)
-
- Analytical Fact Sheet What are the leading causes of death in the African Region? DAK Team; 2023.
-
- Bloom BR. Vaccines for the Third World. Nature. 1989;342(6246):115–120. 1989 342. - PubMed
-
- O’Hagan DT, Singh M, Gupta RK. Poly(lactide-co-glycolide) microparticles for the development of single-dose controlled-release vaccines. Adv Drug Deliv Rev. 1998;32:225–246. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

