PET-based immunomapping of intratumoral CD4+ cells to monitor acquired resistance to checkpoint inhibitors
- PMID: 40561008
- PMCID: PMC12190014
- DOI: 10.1126/sciadv.adw1924
PET-based immunomapping of intratumoral CD4+ cells to monitor acquired resistance to checkpoint inhibitors
Abstract
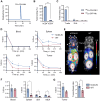
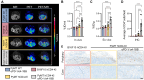
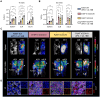
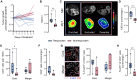
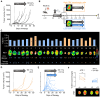
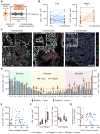
CD4+ T cells are crucial in shaping response and resistance to immunotherapy. To enhance our understanding of their multifaceted functions, we developed copper-64-radiolabeled nanobodies targeting the human CD4 receptor (64Cu-CD4-Nb1) for positron emission tomography (PET). In human CD4-receptor knock-in mice, 64Cu-CD4-Nb1 specifically accumulated in different orthotopic tumors, correlating with histological CD4+ cell densities. Based on intratumoral CD4+ cell distribution patterns within the core and periphery, we distinguished responders to combined αPD-1/4-1BB antibodies early on-treatment. CD4-PET identified resistance to αPD-1 monotherapy, which was mitigated by adding regulatory T cell-depleting α4-1BB antibodies. Patients with early-stage non-small cell lung cancer who relapsed after neoadjuvant αPD-L1 therapy revealed low CD4+ T cell densities in the tumor core. In human and mouse tumor tissues, regulatory T cells correlated with CD4+ cell densities. Thus, visualizing the spatial distribution patterns of CD4+ cells by PET offers mechanistic insights into CD4-mediated therapy efficacy, with great potential for guiding combinatorial immunotherapies in patients with cancer.
Figures
References
-
- Speiser D. E., Chijioke O., Schaeuble K., Münz C., CD4+ T cells in cancer. Nat. Cancer 4, 317–329 (2023). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

