External strain on the plasma membrane is relayed to the endoplasmic reticulum by membrane contact sites and alters cellular energetics
- PMID: 40561024
- PMCID: PMC12190009
- DOI: 10.1126/sciadv.ads6132
External strain on the plasma membrane is relayed to the endoplasmic reticulum by membrane contact sites and alters cellular energetics
Abstract
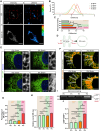
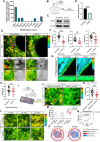
Mechanotransduction is essential for living cells to adapt to their extracellular environment. However, it is unclear how the biophysical adaptation of intracellular organelles responds to mechanical stress or how these adaptive changes affect cellular homeostasis. Here, using the tendon cell as a mechanosensitive cell type within a bioreactor, we show that the tension of the plasma membrane (PM) and the endoplasmic reticulum (ER) adaptively increases in response to repetitive external stimuli. Depletion of stromal interaction molecule 1 (STIM1), the highest expressed PM-ER tether protein, interfered with mechanotransduction from the PM to the ER, and affected the ER tension. We found that an optimized mechanical strain increased ER tension in a homeostatic manner, but excessive strain resulted in ER expansion, as well as activating ER stress. Last, we showed that changes in ER tension were linked with ER-mitochondria interactions and associated with cellular energetics and function. Together, these findings identify a PM-ER mechanotransduction mechanism that dose-dependently regulates cellular metabolism.
Figures







References
-
- Mathieu S., Manneville J. B., Intracellular mechanics: Connecting rheology and mechanotransduction. Curr. Opin. Cell Biol. 56, 34–44 (2019). - PubMed
-
- Ma S., Cahalan S., LaMonte G., Grubaugh N. D., Zeng W., Murthy S. E., Paytas E., Gamini R., Lukacs V., Whitwam T., Loud M., Lohia R., Berry L., Khan S. M., Janse C. J., Bandell M., Schmedt C., Wengelnik K., Su A. I., Honore E., Winzeler E. A., Andersen K. G., Patapoutian A., Common PIEZO1 allele in African populations causes RBC dehydration and attenuates plasmodium infection. Cell 173, 443–455.e12 (2018). - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

