Mechanochemical patterning localizes the organizer of a luminal epithelium
- PMID: 40561030
- PMCID: PMC12190006
- DOI: 10.1126/sciadv.adu2286
Mechanochemical patterning localizes the organizer of a luminal epithelium
Abstract
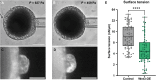
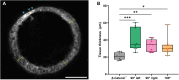
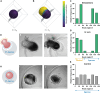
The spontaneous emergence of tissue patterns is often attributed to biochemical reaction-diffusion systems. In Hydra tissue regeneration, the formation of a Wnt signaling center exemplifies this process. However, a strictly biochemical mechanism for self-organization in Hydra remains elusive. In this study, we investigated mechanical stimuli and identified a positive feedback loop between Wnt signaling and tissue stretching. We developed a mathematical model of mechanochemical pattern formation in a closed elastic shell, representing regenerating Hydra epithelial spheroids. Our model explains how mechanical forces drive axis formation and predict the organizer's location under various perturbations. Validation by partially confining regenerating tissues showed that the organizer forms in regions with the greatest stretching potential. This work highlights a versatile mechanochemical mechanism for luminal epithelium patterning, which is relevant across various biological systems.
Figures



References
MeSH terms
LinkOut - more resources
Full Text Sources

