Ubiquitin-dependent proteolysis of KNL2 driven by APC/CCDC20 is critical for centromere integrity and mitotic fidelity
- PMID: 40561118
- PMCID: PMC12231567
- DOI: 10.1093/plcell/koaf164
Ubiquitin-dependent proteolysis of KNL2 driven by APC/CCDC20 is critical for centromere integrity and mitotic fidelity
Abstract
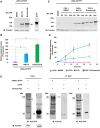
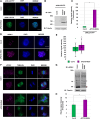
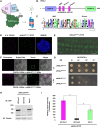
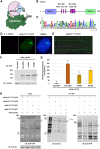
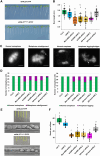
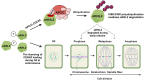
Kinetochores are large protein complexes that serve as attachment sites for spindle microtubules, ensuring proper chromosome segregation during cell division. KINETOCHORE NULL2 (αKNL2) is a key kinetochore protein required for the incorporation of the centromeric histone variant CENH3. The precise regulation of αKNL2 levels is crucial, but the molecular mechanisms controlling this process remain largely unexplored. In this study, we demonstrated that the Anaphase-Promoting Complex/Cyclosome (APC/C) mediates the ubiquitin-dependent proteolysis of αKNL2 during mitosis. Our findings revealed that αKNL2 accumulates in the presence of 26S proteasome inhibitors, and our yeast 2-hybrid and proteomic screens showed that proteins from the ubiquitin-proteasome pathway interact with KNL2 in Arabidopsis (Arabidopsis thaliana) and nematode (Caenorhabditis elegans). Arabidopsis αKNL2 directly interacts with Anaphase-Promoting Complex subunit 10 (APC10) and Cell Division Cycle 20.1 (CDC20.1), 2 substrate recognition components of the APC/C. RNAi-mediated depletion of APC/C resulted in the accumulation and mislocalization of endogenous αKNL2. Additionally, mutation or deletion of the D-box1 region, or substitution of residues K336 and K339, impaired αKNL2 degradation. The expression of a proteasome-resistant αKNL2 variant in planta caused severe defects in growth, fertility, and mitotic division. These findings show that APC/CCDC20-mediated degradation of αKNL2 is critical for proper kinetochore function and centromere integrity.
© The Author(s) 2025. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures


References
-
- Ahmadli U, Kalidass M, Khaitova LC, Fuchs J, Cuacos M, Demidov D, Zuo S, Pecinkova J, Mascher M, Ingouff M, et al. High temperature increases centromere-mediated genome elimination frequency and enhances haploid induction in Arabidopsis. Plant Commun. 2023:4(3):100507. 10.1016/j.xplc.2022.100507 - DOI - PMC - PubMed
-
- Antosz W, Pfab A, Ehrnsberger HF, Holzinger P, Köllen K, Mortensen SA, Bruckmann A, Schubert T, Längst G, Griesenbeck J, et al. The composition of the Arabidopsis RNA polymerase II transcript elongation complex reveals the interplay between elongation and mRNA processing factors. Plant Cell. 2017:29(4):854–870. 10.1105/tpc.16.00735 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

