Neuropathic Pain and Distinct CASPR2 Autoantibody IgG Subclasses Drive Neuronal Hyperexcitability
- PMID: 40561371
- PMCID: PMC12202018
- DOI: 10.1212/NXI.0000000000200423
Neuropathic Pain and Distinct CASPR2 Autoantibody IgG Subclasses Drive Neuronal Hyperexcitability
Abstract
Background and objectives: Patients with autoantibodies (aAbs) against the contactin-associated protein-like 2 (CASPR2) suffer from a variety of clinical syndromes including neuropathic pain. CASPR2 is an adhesion protein of the neurexin family and part of the voltage-gated potassium channel complex (VGKC complex) in dorsal root ganglia (DRG) neurons. The pathologic mechanisms following the binding of CASPR2 aAbs and their association with pain are only partially understood. CASPR2 aAbs are mainly of the IgG4 subclass; however, previous studies have neglected subclass-dependent effects.
Methods: We investigated 49 subclassified patient serum samples positive for CASPR2 aAbs combining superresolution lattice structural illumination microscopy (SIM2) and functional readouts by calcium imaging and electrophysiologic recordings on cultured DRG neurons. CASPR2-positive patient sera subclassified in IgG4 together with at least 1 other IgG subclass (IgGX) and patients with only IgG4 were further subdivided into the pain and no pain groups.
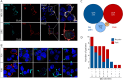
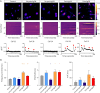
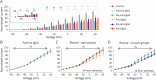
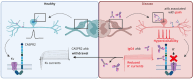
Results: A decrease of CASPR2 expression along the axons after exposure to CASPR2 aAbs was observed for all patient groups except the group without pain and IgG4. Moreover, binding of CASPR2 aAbs from patients with pain increased the distance between CASPR2 and associated potassium channels along DRG axons determined by SIM2 microscopy. CASPR2 aAbs of patients with pain significantly increased overall neuronal excitability of cultured DRG neurons as measured by calcium imaging. Patch-clamp recordings revealed significantly decreased current amplitudes of voltage-gated potassium (Kv) channels after incubation with all 4 CASPR2 aAb subclassifications with the most prominent effect of serum samples harboring IgG4 aAbs only. Replacement of patient aAbs by healthy control serum rescued Kv channel function to normal levels suggesting that the affected potassium channel function is due to structural blockage and disrupted interactions within the VGKC complex. The last might also be rescued on novel protein synthesis and membrane trafficking of CASPR2.
Discussion: IgG4 aAbs seem to be the major modifier of potassium channel function. The DRG hyperexcitability is primarily due to impaired Kv channel conductance as a consequence of CASPR2 aAb binding. However, additional unidentified signal pathways contribute to this process in patients with neuropathic pain.
Conflict of interest statement
The authors report no relevant disclosures. Full disclosure form information provided by the authors is available with the full text of this article at
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
