Ruxolitinib in Patients With Corticosteroid-Refractory or Corticosteroid-Dependent Chronic Graft-Versus-Host Disease: 3-Year Final Analysis of the Phase III REACH3 Study
- PMID: 40561385
- PMCID: PMC12316163
- DOI: 10.1200/JCO-24-02477
Ruxolitinib in Patients With Corticosteroid-Refractory or Corticosteroid-Dependent Chronic Graft-Versus-Host Disease: 3-Year Final Analysis of the Phase III REACH3 Study
Abstract
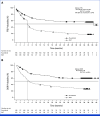
In REACH3 (ClinicalTrials.gov identifier: NCT03112603), ruxolitinib was investigated versus best available therapy (BAT) for 3 years in patients with steroid-refractory/dependent chronic graft-versus-host-disease (SR/D-cGVHD). Patients received ruxolitinib (10 mg twice daily) or BAT for 24 weeks; thereafter (weeks 24-156), patients continued randomized treatment, entered long-term survival follow-up, or crossed over from BAT to ruxolitinib. In 329 randomly assigned patients (ruxolitinib: 165; BAT: 164), the median failure-free survival (FFS) was 38.4 months for ruxolitinib versus 5.7 months for BAT (hazard ratio, 0.36 [95% CI, 0.27 to 0.49]). Median duration of response (DOR) was not reached for ruxolitinib versus 6.4 months for BAT. Ruxolitinib-treated patients had a higher probability of FFS (ruxolitinib: 56.5%; BAT: 18.2%) and maintaining a response (ruxolitinib: 59.6%; BAT: 26.7%) at 36 months. Median overall survival was not reached. Nonrelapse mortality and malignancy relapse/recurrence events were low. In 70 patients who crossed over to ruxolitinib, the overall response rate (50.0%) at week 24 and best overall response (81.4%) during the crossover period were consistent with the primary analysis of randomly assigned patients. No new safety signals were observed. Ruxolitinib provided longer FFS and DOR than BAT, demonstrating sustained efficacy and manageable safety over 3 years of follow-up in patients with SR/D-cGVHD.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures

References
-
- Penack O, Marchetti M, Aljurf M, et al. Prophylaxis and management of graft-versus-host disease after stem-cell transplantation for haematological malignancies: Updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2024;11:e147–e159. - PubMed
-
- Zeiser R, Polverelli N, Ram R, et al. Ruxolitinib for glucocorticoid-refractory chronic graft-versus-host disease. N Engl J Med. 2021;385:228–238. - PubMed
-
- Novartis Pharmaceuticals . Jakavi (Ruxolitinib) Summary of Product Characteristics. https://www.ema.europa.eu/en/documents/product-information/jakavi-epar-p...
-
- Novartis Pharmaceuticals . Jakavi (Ruxolitinib) Prescribing Information. https://www.jakafi.com/pdf/prescribing-information.pdf
-
- Lee SJ, Wolff D, Kitko C, et al. Measuring therapeutic response in chronic graft-versus-host disease. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. The 2014 Response Criteria Working Group report. Biol Blood Marrow Transplant. 2015;21:984–999. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

