Targeting synthetic lethality between non-homologous end joining and radiation in very-high-risk medulloblastoma
- PMID: 40562042
- PMCID: PMC12281405
- DOI: 10.1016/j.xcrm.2025.102202
Targeting synthetic lethality between non-homologous end joining and radiation in very-high-risk medulloblastoma
Abstract
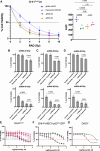
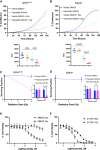
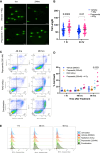
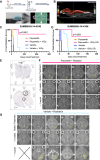
Specific and biologically informed treatments for medulloblastoma, especially for the highly lethal TP53-mutant SHH subgroup, remain elusive, where radiotherapy is the primary treatment modality. Leveraging genome-wide CRISPR-Cas9 dropout screening in combination with lethal doses of radiotherapy, we identify loss of p53 as the main driver of radiation resistance in SHH medulloblastoma. A negative-selection CRISPR-Cas9 screen across multiple models of Trp53-deficient SHH medulloblastoma reveals a strong synthetic lethal interaction between components of the non-homologous end-joining pathway and radiation, particularly DNA-dependent protein kinase (DNA-PK) and its binding partners. Both genetic and pharmacological perturbation of DNA-PK enhance radiosensitivity in TP53-deficient SHH medulloblastoma, leading to cell death. In vivo treatment of both somatic and germline TP53-mutant SHH medulloblastoma models with peposertib, a small-molecule inhibitor of DNA-PK, significantly improves survival when combined with radiotherapy, strongly supporting further clinical investigation.
Keywords: DNA repair; DNA-protein kinase; medulloblastoma; non-homologous end joining; p53; peposertib; radiation; subgroup.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
-
- Leary S.E.S., Packer R.J., Li Y., Billups C.A., Smith K.S., Jaju A., Heier L., Burger P., Walsh K., Han Y., et al. Efficacy of Carboplatin and Isotretinoin in Children With High-risk Medulloblastoma: A Randomized Clinical Trial From the Children's Oncology Group. JAMA Oncol. 2021;7:1313–1321. doi: 10.1001/jamaoncol.2021.2224. - DOI - PMC - PubMed
-
- Michalski J.M., Janss A.J., Vezina L.G., Smith K.S., Billups C.A., Burger P.C., Embry L.M., Cullen P.L., Hardy K.K., Pomeroy S.L., et al. Children's Oncology Group Phase III Trial of Reduced-Dose and Reduced-Volume Radiotherapy With Chemotherapy for Newly Diagnosed Average-Risk Medulloblastoma. J. Clin. Oncol. 2021;39:2685–2697. doi: 10.1200/jco.20.02730. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

