Brexanolone, zuranolone and related neurosteroid GABAA receptor positive allosteric modulators for postnatal depression
- PMID: 40562419
- PMCID: PMC12197409
- DOI: 10.1002/14651858.CD014624.pub2
Brexanolone, zuranolone and related neurosteroid GABAA receptor positive allosteric modulators for postnatal depression
Abstract
Background: Postnatal depression - depression that occurs up to one year after a woman has given birth - is an important and common disorder that can have short- and long-term adverse impacts on the mother, her child and the family as a whole. Recommended treatment for postnatal depression is psychological therapy, and for more severe depression, antidepressants. However, many antidepressants are associated with limited response. Neurosteroid gamma-aminobutyric acid (GABAA) receptor positive allosteric modulators have been developed for the treatment of depression, including postnatal depression, and have a different mechanism of action than traditional antidepressants.
Objectives: To assess the benefits and harms of brexanolone, zuranolone and related neurosteroid GABAA receptor positive allosteric modulators compared to another active treatment (pharmacological, psychological or psychosocial), placebo or treatment as usual for postnatal depression.
Search methods: We searched Cochrane Common Mental Disorders' Specialised Register, CENTRAL, MEDLINE, Embase and PsycINFO in January 2024. We also searched two international trials registries and contacted experts in the field to identify the studies that are included in the review.
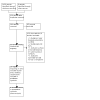
Selection criteria: We included randomised controlled trials (RCTs) of women with depression during the first 12 months following childbirth that compared neurosteroid GABAA receptor positive allosteric modulators with any other treatment (pharmacological, psychological or psychosocial), placebo or treatment as usual.
Data collection and analysis: We used standard Cochrane methodological procedures. The primary outcomes were depression response, depression remission and adverse events experienced by the mother, nursing baby, or both. The secondary outcomes were depression severity, treatment acceptability, quality of life and parenting- and child-related outcomes. We grouped analyses according to whether the neurosteroid GABAA receptor positive allosteric modulator was intravenous or oral. We assessed the certainty of the evidence using GRADE criteria.
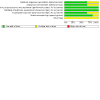
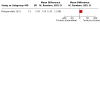
Main results: We identified six RCTs (674 women); all were placebo-controlled trials. Three studies tested intravenous brexanolone; one, intravenous ganaxolone; and two studies, oral zuranolone. Sample sizes ranged from 21 to 196. All were conducted in the USA. We judged the risks of selection, performance, detection, attrition and reporting biases to mostly be low, although the risk of selection and attrition bias was unclear in two studies. The biopharmaceutical companies which made the drugs sponsored all six included studies. They appear to have had a considerable role in the design and conduct of the studies. Intravenous neurosteroid GABAA receptor positive allosteric modulators versus placebo Low-certainty evidence suggests there may be little or no difference in depression response (risk ratio (RR) 1.24, 95% confidence interval (CI) 0.74 to 2.06; I2 = 78%; 3 studies, 267 women) or remission (RR 1.18, 95% CI 0.59 to 2.38; I2 = 73%; 3 studies, 267 women) at 30 days (classified in this review as the 'early phase' of treatment: between 0 and 5 weeks from commencement of treatment). There is also probably little or no difference in the number of adverse events affecting the mother (RR 1.02, 95% CI 0.71 to 1.48; I2 = 46%; 4 studies, 325 women; moderate-certainty evidence). There is low-certainty evidence that there may be little or no difference in depression severity (mean difference (MD) -4.22, 95% CI -8.46 to 0.02; I2 = 78%; 3 studies, 267 women) in the early phase (at 30 days following commencement of treatment); Hamilton Rating Scale for Depression (HAMD-17) score range 0 to 52. Moderate-certainty evidence suggests lower acceptability than placebo, leading to study dropout (RR 2.77, 95% CI 1.22 to 6.26; I2 = 0%; 3 studies, 267 women). No studies measured quality of life or parenting- and child-related outcomes. Oral zuranolone versus placebo Moderate-certainty evidence suggests that zuranolone is probably associated with an improvement in depression response (RR 1.26, 95% CI 1.03 to 1.55; I2 = 13%; 2 studies, 349 women) and remission (RR 1.65, 95% CI 1.22 to 2.22; I2 = 0%; 2 studies, 349 women) at 45 days from commencement of treatment. Moderate-certainty evidence also suggests that zuranolone probably increases the rate of maternal adverse events (RR 1.24, 95% CI 1.03 to 1.48; I2 = 0%; 2 studies, 349 women), when all adverse events are considered; the most frequent adverse event was somnolence. Zuranolone is also probably effective in reducing depression severity at day 45 (MD -3.79, 95% CI -5.60 to -1.97; I2 = 0%; 2 studies, 349 women; moderate-certainty evidence); HAMD-17 score range 0 to 52. Low-certainty evidence suggests little or no difference in terms of treatment acceptability between zuranolone and placebo (RR 0.95, 95% CI 0.50 to 1.81; I2 = 5%; 2 studies, 349 women). No studies measured quality of life. One study reported the Barkin Index of Maternal Functioning (a validated measure of patient-reported maternal functioning within the first year of childbirth), and found that zuranolone improved maternal functioning at day 45 (MD 7.20, 95% CI 1.42 to 12.98; 153 women), but the certainty of this evidence was low.
Authors' conclusions: This review provides moderate-certainty evidence that zuranolone probably improves depression response and remission but also increases maternal adverse events compared to placebo. There may be little or no difference in depression response and remission and probably little or no difference in maternal adverse events with intravenous neurosteroid GABAA positive allosteric modulators such as brexanolone, compared to placebo. Evidence from this review, alongside current clinical guidelines and reference to evidence from the general adult population, could be used to inform an individualised risk-benefit discussion with women seeking treatment for postnatal depression. However, it is difficult to make recommendations about the use of neurosteroid GABAA receptor positive allosteric modulators for the treatment of postnatal depression as no studies have compared them to active treatment.
Copyright © 2025 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
All authors declare no conflicts of interest. Authors employed by Cochrane were not involved in the editorial process for this review.
Figures




















Update of
- doi: 10.1002/14651858.CD014624
Similar articles
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Antidepressant treatment for postnatal depression.Cochrane Database Syst Rev. 2014 Sep 11;2014(9):CD002018. doi: 10.1002/14651858.CD002018.pub2. Cochrane Database Syst Rev. 2014. PMID: 25211400 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
References
References to studies included in this review
Deligiannidis 2021 {published data only}
-
- Clayton A, Cutler AJ, Deligiannidis KM, Lasser R, Sankoh AJ, Doherty J, et al. Clinical efficacy of a 2-week treatment course of Zuranolone for the treatment of major depressive disorder and postpartum depression: outcomes from the clinical development program. In: European Psychiatry Conference: 30th European Congress of Psychiatry, EPA 2022. Virtual. 65(Suppl 1):S97-S98. 2022.
-
- Clayton AH, Deligiannidis KM, Kotecha M, Doherty J. Overview of the rapid antidepressant effects observed in the Zuranolone Clinical Development Program. Obstetrics and Gynecology 2022;139(1):47S-48S.
Deligiannidis 2023 {published data only}
-
- Clayton AH, Deligiannidis KM, Ramos-Quiroga JA, Lasser R, Sankoh AJ, Leclair B, et al. Rapid improvements in MADRS with zuranolone in major depressive disorder and postpartum depression: results from the LANDSCAPE/NEST clinical development programmes. European Psychiatry 2023;66(1):S93-4.
-
- Deligiannidis KM, Meltzer-Brody S, Maximos B, Peeper EQ, Freeman M, Lasser R, Bullock A, et al. Zuranolone for the treatment of postpartum depression. American Journal of Psychiatry 2023;180(9):668-75. - PubMed
-
- EUCTR2020-001424-34-GB. A randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of SAGE-217 in the treatment of adults with severe postpartum depression. clinicaltrialsregister.eu (first received 23 November 2020).
-
- NCT04442503. A study to evaluate the efficacy and safety of SAGE-217 in participants with severe postpartum depression (PPD). clinicaltrials.gov/study/NCT04442503.
Kanes 2017 {published data only}
-
- NCT02614547. A study to evaluate SAGE-547 in patients with severe postpartum depression [A multicenter, randomized, double-blind, parallel-group, placebo-controlled study evaluating the efficacy, safety, and pharmacokinetics of SAGE-547 injection in the treatment of adult female subjects with severe postpartum depression]. ClinicalTrials.gov/show/NCT02614547 (first received 25 November 2015).
Meltzer‐Brody 2018 Study 1 {published data only}
-
- Department of Error. Published Erratum. Lancet 2018;392(10153):1116. [DOI: 10.1016/S0140-6736(18)32288-8] - DOI
-
- Meltzer-Brody S, Colquhoun H, Riesenberg R, Epperson CN, Deligiannidis KM, Rubinow DR, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet 2018;392(10152):1058-70. [DOI: 10.1016/S0140-6736(18)31551-4] - DOI - PubMed
-
- NCT02942004. A study to evaluate efficacy and safety of SAGE-547 in participants with severe postpartum depression (547-PPD-202B) [A multicenter, randomized, double-blind, parallel-group, placebo-controlled study evaluating the efficacy, safety, and pharmacokinetics of SAGE-547 injection in the treatment of adult female subjects with severe postpartum depression and adult female subjects with moderate postpartum depression]. clinicaltrials.gov/ct2/show/NCT02942004 (first received 21 October 2016).
Meltzer‐Brody 2018 Study 2 {published data only}
-
- Department of Error. Published Erratum. Lancet 2018;392(10153):1116. [DOI: 10.1016/S0140-6736(18)32288-8] - DOI
-
- Meltzer-Brody S, Colquhoun H, Riesenberg R, Epperson CN, Deligiannidis KM, Rubinow DR, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet 2018;392(10152):1058-70. [DOI: 10.1016/S0140-6736(18)31551-4] - DOI - PubMed
-
- NCT02942017. A study to evaluate safety and efficacy of SAGE-547 in participants with moderate postpartum depression (547-PPD-202C) [A multicenter, randomized, double-blind, parallel-group, placebo-controlled study evaluating the efficacy, safety, and pharmacokinetics of SAGE-547 injection in the treatment of adult female subjects with severe postpartum depression and adult female subjects with moderate postpartum depression]. clinicaltrials.gov/ct2/show/NCT02942017 (first received 21 October 2016).
NCT03228394 {published data only}
-
- Gutierrez-Esteinou R, Maximos B, Riesenberg R, Johnson KA, Aimetti A, Lappalainen J, et al. Safety and efficacy of intravenous ganaxolone in severe postpartum depression: results from a double-blind, placebo-controlled phase 2 study. Biological Psychiatry 2019;85(10 Suppl):S181-2.
-
- NCT03228394. A clinical trial of intravenous ganaxolone in women with postpartum depression [A phase 2a, double-blind, placebo-controlled, multiple-dose escalation study to evaluate safety, pharmacokinetics and efficacy of intravenously administered ganaxolone in women with postpartum depression]. clinicaltrials.gov/ct2/show/NCT03228394 (first received 24 July 2017).
References to studies excluded from this review
Clayton 2020 {published data only}
-
- Clayton A, Lasser R, Nandy I, Sankoh A, Campbell A, Werneburg B, et al. A phase 3, multicenter, double-blind, randomized, placebo-controlled study evaluating the efficacy of SAGE-217 in the treatment of adult patients with major depressive disorder. Biological Psychiatry 2020;87(9):S86.
-
- NCT03672175. A study to evaluate the efficacy of SAGE-217 in the treatment of adult subjects with major depressive disorder [A phase 3, multicenter, double-blind, randomized, placebo-controlled study evaluating the efficacy of SAGE-217 in the treatment of adult subjects with major depressive disorder]. clinicaltrials.gov/ct2/show/NCT03672175 (first received 14 September 2018).
Gunduz‐Bruce 2019 {published data only}
-
- Bonthapally V, Gunduz-Bruce H, Silber C, Rothschild A, Riesenberg R, Sankoh A, et al. Health-related quality of life in a phase 2, randomized, placebo-controlled trial of the GABA(A)R modulator SAGE-217 in major depressive disorder. European Neuropsychopharmacology 2019;29:S58.
-
- Gunduz-Bruce H, Silber C, Kaul I, Rothschild AJ, Riesenberg R, Sankoh AJ, et al. Trial of SAGE-217 in patients with major depressive disorder. New England Journal of Medicine 2019;381(10):903-11. [10.1056/NEJMoa1815981] - PubMed
-
- Gunduz-Bruce H, Silber C, Rothschild A, Riesenberg R, Sankoh A, Li H, et al. SAGE-217 a first in class GABAA receptor positive allosteric modulator in major depressive disorder: a multicenter, randomized, double-blind, phase 2 placebo-controlled trial. Biological Psychiatry 2018;83(9):S127-8.
-
- Gunduz-Bruce H, Silber C, Rothschild A, Riesenberg R, Sankoh A, Li H, et al. SAGE-217 in major depressive disorder: a multicenter, randomized, double-blind, phase 2 placebo-controlled trial. European Neuropsychopharmacology 2019;29:S59-60.
-
- NCT03000530. A study to evaluate sage-217 in participants with moderate to severe major depressive disorder [A phase 2, two-part (open-label followed by double-blind) study evaluating the safety, tolerability, pharmacokinetics, and efficacy of sage-217 in the treatment of adult subjects with moderate to severe major depressive disorder]. clinicaltrials.gov/ct2/show/NCT03000530 (first received 22 December 2016).
Hoffmann 2019 {published data only}
-
- Hoffmann E, Wald J, Colquhoun H. 850: Evaluation of breast milk concentrations following brexanolone iv administration to healthy lactating women. American Journal of Obstetrics and Gynecology 2019;220(Suppl 1):S554. [DOI: 10.1016/j.ajog.2018.11.873] - DOI
-
- Hoffmann E, Wald J, Dray D, Colquhoun H. Brexanolone injection administration to lactating women: breast milk allopregnanolone levels. In: Obstetrics and Gynecology. Conference: 67th Annual Clinical and Scientific Meeting of the American College of Obstetricians and Gynecologists. Vol. 133. Nashville, TN, 2019:Suppl 1.
NCT03460756 {published data only}
-
- NCT03460756. A clinical trial of oral ganaxolone in women with postpartum depression [A phase 2, double-blind, placebo-controlled, multicenter study to evaluate safety, tolerability, and efficacy of oral administration of ganaxolone in women with postpartum depression]. clinicaltrials.gov/ct2/show/NCT03460756 (first received 9 March 2018).
NCT03864614 {published data only}
-
- NCT03864614. A study to evaluate SAGE-217 in adult participants with major depressive disorder (MDD) [A Phase 3, open-label, 1-year study of the safety, tolerability, and need for re-treatment with SAGE-217 in adult subjects with major depressive disorder]. clinicaltrials.gov/ct2/show/NCT03864614 (first received 6 March 2019).
NCT03924492 {published data only}
-
- NCT03924492. Expanded access protocol of Zulresso™ (brexanolone) injection for adult patients with postpartum depression [Expanded access protocol of Zulresso for adult patients with postpartum depression]. clinicaltrials.gov/ct2/show/NCT03924492 (first received 18 February 2020).
NCT04273191 {published data only}
-
- NCT04273191. A study to evaluate multimodal neuroimaging parameters in women with postpartum depression who are receiving Zulresso™ (brexanolone) [An evaluation of multimodal neuroimaging parameters in women with postpartum depression who are receiving Zulresso]. clinicaltrials.gov/ct2/show/NCT04273191 (first received 18 February 2020).
NCT04442490 {published data only}
-
- NCT04442490. A study to evaluate the efficacy of SAGE-217 in the treatment of adult participants with major depressive disorder (MDD) [A Phase 3, multicenter, double-blind, randomized, placebo-controlled study evaluating the efficacy of SAGE-217 in the treatment of adult subjects with major depressive disorder]. clinicaltrials.gov/ct2/show/NCT04442490 (first received 2 June 2020).
Riesenberg 2022 {published data only}
-
- NCT03665038. A study to assess the safety of brexanolone in the treatment of adolescent female participants with postpartum depression (PPD) [A multicenter, open-label study evaluating the safety, tolerability, and pharmacokinetics of brexanolone in the treatment of adolescent female subjects with postpartum depression]. clinicaltrials.gov/ct2/show/NCT03665038 (first received 11 September 2018).
-
- Riesenberg R, Bankole K, Garafola S, Qin M, Hoffmann E, Brown C, et al. Brexanolone in adolescent patients with postpartum depression: results from the phase 3, open-label CHICKADEE study. American Journal of Obstetrics and Gynecology 2022;226(1 Supplement):S79. [DOI: 10.1016/j.ajog.2021.11.149] - DOI
References to studies awaiting assessment
Reisdorf 2021 {published data only}
-
- Reisdorf S. Postpartum depression. Zuranolone improves HAM-D-17 score during and after 2-week therapy. Krankenhauspharmazie 2021;42(11):481-2.
Additional references
APA 2013
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM–5). 5th edition. Washington, DC: American Psychiatric Association, 2013.
Bland 2000
Brown 2021
Cooper 1995
-
- Cooper PJ, Murray L. Course and recurrence of postnatal depression. Evidence for the specificity of the diagnostic concept. British Journal of Psychiatry 1995;166(2):191-5. - PubMed
Cooper 2019
-
- Cooper MC, Kilvert HS, Hodgkins P, Roskell NS, Eldar-Lissai A. Using matching-adjusted indirect comparisons and network meta-analyses to compare efficacy of brexanolone injection with selective serotonin reuptake inhibitors for treating postpartum depression. CNS Drugs 2019;33:1039-52. - PMC - PubMed
Covidence [Computer program]
-
- Covidence. Version accessed 10 December 2019. Melbourne, Australia: Veritas Health Innovation, 2019. Available at covidence.org.
Cox 1987
-
- Cox J, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. British Journal of Psychiatry 1987;150:782-6. - PubMed
Deeks 2023
-
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023. Available from www.training.cochrane.org/handbook.
Deligiannidis 2019
Epperson 2006
-
- Epperson CN, Gueorguieva R, Czarkowski KA, Stiklus S, Sellers E, et al. Preliminary evidence of reduced occipital GABA concentrations in puerperal women: a 1H-MRS study. Psychopharmacology 2006;186:425. - PubMed
Gerbasi 2021
-
- Gerbasi ME, Kosinski M, Meltzer-Brody S, Acaster S, Fridman M, Huang MY, et al. Achieving clinical response in postpartum depression leads to improvement in health-related quality of life. Current Medical Research and Opinion 2021;37(7):1221-31. - PubMed
Goodman 2004
-
- Goodman JH. Postpartum depression beyond the early postpartum period. Journal of Obstetrics, Gynecology and Neonatal Nursing 2004;33:410-20. - PubMed
GRADEpro GDT 2015 [Computer program]
-
- GRADEpro GDT. Version accessed prior to 3 March 2020. McMaster University (developed by Evidence Prime), 2020. Available at gradepro.org.
Grigoriadis 2017
Hamilton 1967
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from https://training.cochrane.org/handbook/archive/v5.1/.
Higgins 2023
-
- Higgins JP, Li T, Deeks JJ (editors). Chapter 6: Choosing effect measures and computing estimates of effect [last updated August 2023]. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.5. Cochrane, 2024. Available from www.training.cochrane.org/handbook.
Howard 2014
Jones 2014
-
- Jones I, Chandra PS, Dazzan P, Howard LM. Bipolar disorder, affective psychosis, and schizophrenia in pregnancy and the post-partum period. Lancet 2014;384(9956):1789-99. - PubMed
Khalifeh 2016
-
- Khalifeh H, Hunt IM, Appleby L, Howard LM. Suicide in perinatal and non-perinatal women in contact with psychiatric services: 15 year findings from a UK national inquiry. Lancet Psychiatry 2016;3(3):233-42. - PubMed
Knight 2019
-
- Knight M, Bunch K, Tuffnell D, Shakespeare J, Kotnis R, Kenyon S, et al (editors), on behalf of MBRRACE-UK. Saving Lives, Improving Mothers’ Care - Lessons learned to inform maternity care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2015-17. Oxford, UK: National Perinatal Epidemiology Unit, University of Oxford, 2019.
Luisi 2000
-
- Luisi S, Petraglia F, Nappi RE, Bernardi F, Fadalti M, Reis FM, et al. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. Journal of Clinical Endocrinology & Metabolism 2000;85(7):2429-33. - PubMed
Maguire 2019
McAllister‐Williams 2017
-
- McAllister-Williams RH, Baldwin DS, Cantwell R, Easter A, Gilvarry E, Glover V, et al. British Association for Psychopharmacology consensus guidance on the use of psychotropic medication preconception, in pregnancy and postpartum 2017. Journal of Psychopharmacology 2017;31(5):519-52. - PubMed
Meltzer‐Brody 2020
Munk‐Olsen 2006
-
- Munk-Olsen T, Laursen TM, Pedersen CB, Mors O, Mortensen PB. New parents and mental disorders: a population-based register study. JAMA 2006;296(21):2582-9. - PubMed
Munk‐Olsen 2009
-
- Munk-Olsen T, Laursen TM, Mendelson T, Pedersen CB, Mors O, Mortensen PB. Risks and predictors of readmission for a mental disorder during the postpartum period. Archives of General Psychiatry 2009;66(2):189-95. - PubMed
Munk‐Olsen 2016
NICE 2020
-
- National Institute for Health and Care Excellence. Antenatal and postnatal mental health: clinical management and service guidance. Clinical guideline [CG192]; published December 2014; last updated February 2020. Available at www.nice.org.uk/guidance/cg192/resources/antenatal-and-postnatal-mental-....
O'Hara 2013
-
- O'Hara MW, McCabe JE. Postpartum depression: current status and future directions. Annual Review of Clinical Psychology 2013;9:379-407. - PubMed
O'Mahen 2008
-
- O'Mahen HA, Flynn HA. Preferences and perceived barriers to treatment for depression during the perinatal period. Journal of Women's Health 2008;17(8):1301-9. - PubMed
Paoletti 2006
-
- Paoletti AM, Romagnino S, Contu S, Orru MM, Marotto MF, Zedda P, et al. Observational study on the stability of the psychological status during normal pregnancy and increased blood levels of neuroactive steroids with GABA-A receptor agonist activity. Psychoneuroendocrinology 2006;31(4):485-92. - PubMed
RevMan 2024 [Computer program]
-
- Review Manager (RevMan). Version 7.12.0. The Cochrane Collaboration, 2024. Available at https://revman.cochrane.org.
Salwan 2023
Schünemann 2019
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing 'Summary of findings' tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Silverman 2019
Spitzer 1978
-
- Spitzer RL, Endicott J, Robins E. Research diagnostic criteria: rationale and reliability. Archives of General Psychiatry 1978;35(6):773-82. - PubMed
Stein 2014
-
- Stein A, Pearson RM, Goodman SH, Rapa E, Rahman A, McCallum M, et al. Effects of perinatal mental disorders on the fetus and child. Lancet 2014;384(9956):1800-19. - PubMed
Stewart 2016
-
- Stewart DE, Vigod S. Postpartum depression. New England Journal of Medicine 2016;375(22):2177-86. - PubMed
Stewart 2019
-
- Stewart DE, Vigod SN. Postpartum depression: pathophysiology, treatment, and emerging therapeutics. Annual Review of Medicine 2019;70:183-96. - PubMed
Ware 1992
-
- Ware JE Jr, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): I. Conceptual framework and item selection. Medical Care 1992;30(6):473-83. - PubMed
WHO 2018
-
- World Health Organization. International classification of diseases for mortality and morbidity statistics (11th Revision). 18 June 2018. www.who.int/classifications/icd/en/ (accessed prior to 3 March 2020).
Wisner 2004
-
- Wisner KL, Perel JM, Peindl KS, Hanusa BH, Piontek CM, Findling RF. Prevention of postpartum depression: a pilot randomized clinical trial. American Journal of Psychiatry 2004;161(7):1290-2. - PubMed
Wisner 2013
Woody 2017
-
- Woody CA, Ferrari AJ, Siskind DJ, Whiteford HA, Harris MG. A systematic review and meta-regression of the prevalence and incidence of perinatal depression. Journal of Affective Disorders 2017;219:86-92. - PubMed
Xia 2009
-
- Xia J, Adams C, Bhagat N, Bhagat V, Bhoopathi P, El-Sayeh H, et al. Losing participants before the trial ends erodes credibility of findings. Psychiatric Bulletin 2009;33(7):254-7.
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

