Super-resolution upgrade for deep tissue imaging featuring simple implementation
- PMID: 40562760
- PMCID: PMC12198360
- DOI: 10.1038/s41467-025-60744-y
Super-resolution upgrade for deep tissue imaging featuring simple implementation
Abstract
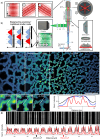
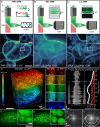
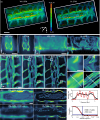
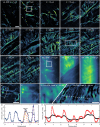
Deep tissue imaging with high contrast close to or even below the optical resolution limit is still challenging due to optical aberrations and scattering introduced by dense biological samples. This results in high complexity and cost of microscopes that can facilitate such challenges. Here, we demonstrate a cost-effective and simple to implement method to turn most two-photon laser-scanning microscopes into a super-resolution microscope for deep tissue imaging. We realize this by adding inexpensive optical devices, namely a cylindrical lens, a field rotator, and a sCMOS camera to these systems. By combining two-photon excitation with patterned line-scanning and subsequent image reconstruction, we achieve imaging of sub-cellular structures in Pinus radiata, mouse heart muscle and zebrafish. In addition, the penetration depth of super-resolved imaging in highly scattering tissue is considerably extended by using the camera's lightsheet shutter mode. The flexibility of our method allows the examination of a variety of thick samples with a variety of fluorescent markers and microscope objective lenses. Thus, with a cost-efficient modification of a multi-photon microscope, an up to twofold resolution enhancement is demonstrated down to at least 70μm deep in tissue.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
References
-
- Schermelleh, L. et al. Super-resolution microscopy demystified. Nat. Cell Biol.21, 72–84 (2019). - PubMed
MeSH terms
Grants and funding
- H.2-F116.MUE/54/2, FSP Angewandte Photonik/Bayerisches Staatsministerium für Bildung und Kultus, Wissenschaft und Kunst (State Ministry of Education and Culture, Science and the Arts)
- DFG 510049865/Deutsche Forschungsgemeinschaft (German Research Foundation)
- SFB TRR 267 (No. 403584255, project B05)/Deutsche Forschungsgemeinschaft (German Research Foundation)
LinkOut - more resources
Full Text Sources

