Outcomes of teclistamab in patients with relapsed/refractory multiple myeloma with prior exposure to BCMA-directed therapy: a multicenter study from the U.S. Multiple Myeloma Immunotherapy Consortium
- PMID: 40562770
- PMCID: PMC12198414
- DOI: 10.1038/s41408-025-01314-9
Outcomes of teclistamab in patients with relapsed/refractory multiple myeloma with prior exposure to BCMA-directed therapy: a multicenter study from the U.S. Multiple Myeloma Immunotherapy Consortium
Abstract
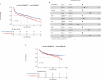
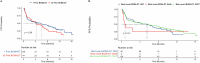
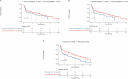
Data describing outcomes of teclistamab in multiple myeloma patients with prior exposure to BCMA-directed therapy (BCMA-DT) are limited. The goal of this multicenter retrospective analysis was to report the efficacy and safety of standard-of-care teclistamab in patients with prior BCMA-DT. A total of 385 patients were included, of whom 193 (50%) had received prior BCMA-DT, including 47 (24%) patients with prior antibody-drug conjugate (ADC)-only, 99 (51%) with chimeric antigen receptor T-cell therapy (CAR T)-only, 36 (19%) with both ADC and CAR T, 6 (3%) with bispecific antibody-only, and 5 (3%) with other combinations. Most safety parameters between cohorts were comparable. The prior BCMA-DT cohort had a lower overall response rate (ORR: 48.7% versus 61.5%; p = 0.012), and median progression-free survival (PFS: 4.6 versus 8.2 months; p = 0.017) compared to the cohort without prior BCMA-DT. However, in multivariable analysis, despite a clear trend, ultimately receipt of a prior BCMA-DT was not independently associated with ORR or PFS (p = 0.057 and p = 0.1, respectively). No significant differences in PFS were noted when stratifying patients by number of prior BCMA-DTs, types of all prior BCMA-DTs received, type of most recent prior BCMA-DT, or depth of response to most recent BCMA-DT. Using the maximally selected rank statistics method, the optimal cut-off for time from the last BCMA-DT exposure to teclistamab initiation was identified as 8.7 months. Patients with >8.7 months between their last exposure to prior BCMA-DT and teclistamab initiation had a significantly improved median PFS with teclistamab (8.1 months, 95% CI: 4.6-11.7) compared to patients with <8.7 months (2.5 months, 95% CI: 1.1-5.7), p = 0.001. Altogether, our findings support the use of teclistamab as a viable treatment option in patients previously exposed to BCMA-DT.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: RB reports consulting: Adaptive Biotech, BMS, Caribou Biosciences, Genentech, Janssen, Karyopharm, Legend Biotech, Pfizer, Sanofi, SparkCures; research: AbbVie, BMS, Janssen, Novartis, Pack Health, Prothena, Sanofi. GK reports consulting: BMS, Arcellx, Sanofi, Janssen, Cellectar, Pfizer, Kedrion; research: BMS, Janssen, AbbVie. SS reports research: Magenta Therapeutics, BMS, Allogene, Janssen, Novartis, AbbVie; Advisory Board/Consultancy: BMS, Janssen, Sanofi, Oncopeptides, Takeda, Regeneron, AbbVie, Pfizer, BiolineRx, Legend, Kite. JAD reports consultancy for BMS, Janssen; speaker’s bureau for Janssen. TR reports consulting: BMS, J&J, Pfizer, Sanofi. SR reports honoraria: Janssen, BMS, Genentech, Karyopharm Therapeutics, MJH LifeSciences; steering committees: Gracell Therapeutics, BMS; research support: Janssen, BMS, C4 Therapeutics, Gracell Therapeutics, Heidelberg Pharma; consulting: Genentech, Janssen, BMS, Karyopharm Therapeutics. LS reports consulting: BMS. JK reports consulting: GPCR, Janssen, Prothena, Legend Biotech; research: Prothena, Ascentage, Janssen, Karyopharm, GPCR. HCL reports consulting: Bristol Myers Squibb, Pfizer, Janssen, Regeneron, GlaxoSmithKline, Sanofi, AbbVie, Takeda Pharmaceuticals, Allogene Therapeutics, Menarini, Alexion Pharmaceuticals; research funding: Amgen, Bristol Myers Squibb, Janssen, GSK, Regeneron, Takeda Pharmaceuticals. KKP reports consulting: BMS, Janssen, AstraZeneca, Legend Biotech, Kite, Genentech, AbbVie, Sanofi, Caribou, Takeda, Regeneron, Poseida. DKH reports research funding from BMS, Janssen, Karyopharm, Kite Pharma, and Adaptive Biotech; Consulting or advisory role for BMS, Janssen, Legend Biotech, Pfizer, Kite Pharma, AstraZeneca, and Karyopharm. AA reports research funding from AbbVie, Adaptive Biotech, K36-therapeutics, J&J, Regeneron Pharmaceuticals; advisory role for Karyopharm, BMC, Sanofi, J&J, Pfizer. CJF reports consulting: Janssen; research: Janssen, Regeneron; ownership of publicly traded stock: Affimed. YL reports consulting: Janssen, Legend, Celgene, Sanofi, BMS, Pfizer, Regeneron, Genentech, NexImmune, Caribou; research funding: Janssen, Celgene, BMS. AJC reports consulting: AbbVie, Adaptive, BMS, HopeAI, Janssen, Sebia, Sanofi; research: AbbVie, Adaptive Biotechnologies, Caelum, Harpoon, Nektar, BMS, Janssen, Sanofi, OpnaBio, IgM Biosciences, Regeneron. LDA Jr reports consulting: Janssen, Celgene, BMS, Amgen, GSK, AbbVie, BeiGene, Cellectar, Sanofi, Prothena; research: BMS, Celgene, GSK, Janssen, AbbVie. ALG reports research funding: Johnson & Johnson, Novartis, Tmunity, CRISPR Therapeutics; consulting: Johnson & Johnson, Gracell, BMS, GSK, Regeneron, AbbVie, SmartImmune; DSMB membership for Johnson & Johnson. The remaining authors have no conflicts to disclose. SSA reports consultancy for Sanofi. HH reports consulting for Janssen; speaker bureaus for Janssen, Karyopharm. FA served as advisor and speaker for BMS, Celgene, Caribou Biosciences; research funding from Allogene Therapeutics, Celgene, GlaxoSmithKline, Bristol Myers Squibb, and Caribou Biosciences. SR reports advisory board for Pfizer, Prothena Biosciences, and KiTE; research funding from Poseida, Therapeutics, Nexcella Inc., and Janssen. AR reports consultancy for Adaptive, BMS, Janssen, Karyopharm, and Sanofi. SA reports research funding: GSK, Amgen, Karyopharm; honoraria: Janssen. DS reports consulting for Sanofi, Janssen, Pfizer, BMS, GlaxoSmithKline, Legend Biotech, Bioline, AstraZeneca, and Arcellx. KHS reports consulting for Aaptive Biotech, Janssen, Takeda, Sanofi, GlaxoSmithKline; research funding: Janssen, BMS, Karyopharm, Amgen. AG-C reports advisory board for Cellectar Biosciences, Janssen, Pfizer; honoraria: Cellectar Biosciences, Janssen, Sanofi, Amgen, Pfizer; research funding: Cellectar Biosciences. OC reports consultancy for Janssen, BMS, Legend Biotech; honoraria: BMS for speaker bureau. PMV reports consultancy for AbbVie, AstraZeneca, BMS, Karyopharm, Lava Therapeutics, Sanofi, Regeneron, Janssen, GSK; research funding: AbbVie, GSK, Janssen, Regeneron. LM reports advisory board with Legend Biotech and BioLineRx. MRG advisory board to BMS and Arcellx. KJ reports advisory board consultant for Janssen, Pfizer, and BioLineRx. The rest of the authors have no conflicts of interest. Ethics approval and consent to participate: Each center obtained Institutional Review Board approval, which included a waiver of informed consent. The study was conducted in compliance with the International Conference on Harmonization (ICH), Good Clinical Practices (GCPs), and the Declaration of Helsinki. The protocol was approved by the respective Institutional Review Board or ethics committee at each of the participating institutions (MD Anderson Cancer Center, Houston, TX; Fred Hutchinson Cancer Center, Seattle, WA; Cleveland Clinic Lerner College of Medicine, Case Western Reserve University School of Medicine, Cleveland, OH; Medical University of South Carolina, Charleston, SC; Myeloma, Waldenstrom’s, and Amyloidosis Program, Simmons Comprehensive Cancer Center, UT Southwestern Medical Center, Dallas, TX; Moffitt Cancer Center, Tampa, FL; Mount Sinai School of Medicine, New York, NY; Stanford University, Palo Alto, CA; Huntsman Cancer Institute, University of Utah, Salt Lake City, UT; Atrium Health Levine Cancer Institute, Wake Forest University School of Medicine, Charlotte, NC; University of Kansas Medical Center, Kansas City, KS; Abramson Cancer Center and Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA; Roswell Park Comprehensive Cancer Center, Buffalo, NY; Duke University Cancer Institute, Durham, NC; Mayo Clinic, Division of Hematology, Rochester, MN; Dana-Farber Cancer Institute, Boston, MA; all in the USA). All methods were performed in accordance with the relevant guidelines and regulations. All authors had access to the data and contributed to the analysis and interpretation of the results. The authors confirm the accuracy and completeness of the data.
Figures

References
-
- Munshi NC, Anderson LD, Shah N, Madduri D, Berdeja J, Lonial S, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384:705–16. - PubMed
-
- Berdeja JG, Madduri D, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021;398:314–24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

