Efficacy, public health impact and optimal use of the Takeda dengue vaccine
- PMID: 40563017
- PMCID: PMC12353809
- DOI: 10.1038/s41591-025-03771-y
Efficacy, public health impact and optimal use of the Takeda dengue vaccine
Abstract
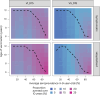
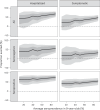
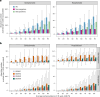
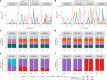
Dengue is the most common arboviral infection, causing substantial morbidity and mortality globally. The licensing of Qdenga, a second-generation vaccine developed by Takeda Pharmaceuticals, is therefore timely, but the potential public health impact of vaccination across transmission settings needs to be evaluated. To address this, we characterized Qdenga's efficacy profile using mathematical models calibrated to published clinical trial data and estimated the public health impact of routine vaccine use. We find that efficacy against both virologically confirmed dengue and hospitalization depends on the infecting serotype, serological status and age. We estimate that vaccination of children aged over 6 years in moderate-to-high dengue transmission settings (average seroprevalence in 9-year-olds > 60%) could reduce the burden of hospitalized dengue by 10-22% on average over 10 years. We find some evidence of a risk of vaccine-induced disease enhancement in seronegative vaccine recipients for dengue serotypes 3 and 4, especially for children under 6 years of age. Because of this, the benefits of vaccination in lower transmission settings are more uncertain, and more data on the long-term efficacy of Qdenga against serotypes 3 and 4 are needed.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

