Copper supplementation mitigates Parkinson-like wild-type SOD1 pathology and nigrostriatal degeneration in a novel mouse model
- PMID: 40563111
- PMCID: PMC12188662
- DOI: 10.1186/s40478-025-02048-2
Copper supplementation mitigates Parkinson-like wild-type SOD1 pathology and nigrostriatal degeneration in a novel mouse model
Abstract
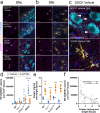
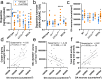
Misfolded wild-type superoxide dismutase 1 (disSOD1) protein is implicated in the death of substantia nigra (SN) dopamine neurons in Parkinson disease. Regionally reduced copper availability, and subsequent reduced copper binding to SOD1, is a key factor driving the development of this pathology, suggesting brain copper supplementation may constitute an effective means of preventing its formation. We evaluated whether the blood-brain-barrier-permeable copper delivery drug, CuATSM, attenuated the misfolding and deposition of wild-type disSOD1 and associated neuron death in a novel mouse model that expresses this pathology. These factors were profiled using proteomic and elemental mass spectrometry, together with biochemical and histological workflows. We demonstrated copper supplementation corrects altered post-translational modifications on soluble SOD1 and improves the enzymatic activity of the protein in the brains of these animals. These changes were associated with a significant reduction in disSOD1 pathology and preservation of dopamine neurons in the SN, which were highly correlated with tissue copper levels. Our data position wild-type disSOD1 pathology as a novel drug target for Parkinson disease and suggest that brain copper supplementation may constitute an effective means of slowing SN dopamine neuron death in this disorder.
Keywords: Copper supplementation; CuATSM; Mouse model; Neurodegeneration; Parkinson disease; Post-translational modification; Protein misfolding; Substantia Nigra Pars compacta; Superoxide dismutase 1.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All experimental procedures involving the use of mice conformed to the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes, with protocols approved by the Animal Ethics Committee at the University of Sydney (Ethics ID: 2020/1849). Consent for publication: All authors read and approved the final manuscript prior to publication. Competing interests: The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

