Prokaryotic mechanosensitive channels mediate copper influx
- PMID: 40563205
- PMCID: PMC12198049
- DOI: 10.1002/pro.70205
Prokaryotic mechanosensitive channels mediate copper influx
Abstract
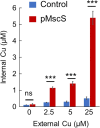
Copper is an essential micronutrient in all kingdoms of life, requiring a meticulous balance between acquisition and toxic overload. While copper import in eukaryotes has been investigated extensively, few prokaryotic copper importers have been identified, leading to the notion that cytoplasmic copper uptake is unnecessary in prokaryotes. Here we report that mechanosensitive channels are key players in prokaryotic copper import. Deletion of the gene encoding the Escherichia coli small mechanosensitive channel, EcMscS, leads to significantly reduced copper influx. Conversely, overexpression of EcMscS leads to increased copper influx, elevated intracellular copper content, and renders cells hypersensitive to copper. Furthermore, specific channel blockers and competing permeating ions inhibit EcMscS copper conductance, lowering intracellular copper accumulation and alleviating copper hypersensitivity. These findings extend beyond E. coli, as other prokaryotic small mechanosensitive channels of bacterial and archaeal origin also facilitate copper influx. Taken together, these results uncover a previously unknown moonlighting function for mechanosensitive channels as a pathway for prokaryotic copper uptake.
Keywords: bacteria; copper; ion channels; mechanosensitive channels; membrane permeation; metal homeostasis; prokaryotes; transport.
© 2025 The Author(s). Protein Science published by Wiley Periodicals LLC on behalf of The Protein Society.
Conflict of interest statement
The authors declare no conflicting interests.
Figures






Update of
-
Prokaryotic mechanosensitive channels mediate copper influx.bioRxiv [Preprint]. 2025 Mar 24:2025.03.24.644891. doi: 10.1101/2025.03.24.644891. bioRxiv. 2025. Update in: Protein Sci. 2025 Jul;34(7):e70205. doi: 10.1002/pro.70205. PMID: 40196475 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

