Lactobacillus rhamnosus GG Modulates Mitochondrial Function and Antioxidant Responses in an Ethanol-Exposed In Vivo Model: Evidence of HIGD2A-Dependent OXPHOS Remodeling in the Liver
- PMID: 40563263
- PMCID: PMC12189657
- DOI: 10.3390/antiox14060627
Lactobacillus rhamnosus GG Modulates Mitochondrial Function and Antioxidant Responses in an Ethanol-Exposed In Vivo Model: Evidence of HIGD2A-Dependent OXPHOS Remodeling in the Liver
Abstract
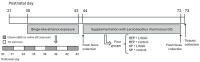
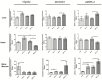
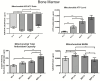
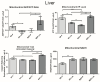
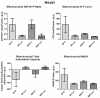
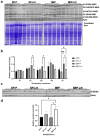
The gut microbiota plays a central role in host energy metabolism and the development of metabolic disorders, partly through its influence on mitochondrial function. Probiotic supplementation, particularly with Lactobacillus rhamnosus GG, has been proposed as a strategy to modulate the microbiota and improve host metabolic health. Adolescent binge-like alcohol consumption is a critical public health issue known to induce neuroinflammation, oxidative stress, mitochondrial dysfunction, and intestinal dysbiosis, contributing to disorders such as alcoholic liver disease (ALD). This study aimed to evaluate the effects of L. rhamnosus GG supplementation on mitochondrial physiology in Sprague Dawley rats exposed to binge-like ethanol (BEP group) or saline (SP group) during adolescence (postnatal days 30-43). Starting on postnatal day 44, L. rhamnosus GG was administered orally for 28 days. Fecal colonization was confirmed by qPCR, and mitochondrial function was assessed in the liver, heart, and bone marrow through quantification of NADH, ATP, ADP/ATP ratio, total antioxidant capacity, and the expression of mitochondrial genes Higd2a, MnSOD1, and AMPKα1. L. rhamnosus GG supplementation induced tissue-specific mitochondrial adaptations. In the liver, it increased Higd2a expression and restored antioxidant and energy balance in ethanol-exposed rats. In the bone marrow, it reversed ethanol-induced metabolic stress and enhanced AMPKα1 expression. In contrast, in the heart, L. rhamnosus GG had minimal impact on mitochondrial energy markers but increased antioxidant capacity, indicating a more limited, redox-focused effect. These findings suggest that L. rhamnosus GG exerts context-dependent, tissue-specific benefits on mitochondrial physiology, primarily through the modulation of antioxidant defenses, activation of AMPKα1, and remodeling of respiratory complexes. This probiotic may represent a promising therapeutic strategy to mitigate mitochondrial dysfunction associated with early-life alcohol exposure.
Keywords: ADP/ATP ratio; AMPKα1; Higd2a; Lactobacillus rhamnosus GG; MnSOD; NADH; OXPHOS; binge-like ethanol exposure; microbiota; mitochondrial physiology.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study, in the collection, analysis, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Gut Microbiome Engineering for Diabetic Kidney Disease Prevention: A Lactobacillus rhamnosus GG Intervention Study.Biology (Basel). 2025 Jun 19;14(6):723. doi: 10.3390/biology14060723. Biology (Basel). 2025. PMID: 40563973 Free PMC article.
-
Lactiplantibacillus plantarum Lp05 protects against ethanol-induced liver injury in zebrafish through metabolic and microbiota modulation.Sci Rep. 2025 Jul 2;15(1):22584. doi: 10.1038/s41598-025-07111-5. Sci Rep. 2025. PMID: 40593078 Free PMC article.
-
Probiotics for management of functional abdominal pain disorders in children.Cochrane Database Syst Rev. 2023 Feb 17;2(2):CD012849. doi: 10.1002/14651858.CD012849.pub2. Cochrane Database Syst Rev. 2023. PMID: 36799531 Free PMC article.
-
Effect of viable and inactivated Lactobacillus rhamnosus GG administration on the prevention of diet-induced obesity in rats: Implication of white and brown adipose tissue and influence of bacterial viability.J Nutr Biochem. 2025 Oct;144:109982. doi: 10.1016/j.jnutbio.2025.109982. Epub 2025 Jun 6. J Nutr Biochem. 2025. PMID: 40482732
-
Synbiotics, prebiotics and probiotics for solid organ transplant recipients.Cochrane Database Syst Rev. 2022 Sep 20;9(9):CD014804. doi: 10.1002/14651858.CD014804.pub2. Cochrane Database Syst Rev. 2022. PMID: 36126902 Free PMC article.
References
-
- Carvajal F., Lerma-Cabrera J.M. Alcohol Consumption Among Adolescents—Implications for Public Health. In: Claborn D.M., editor. Topics in Public Health. IntechOpen; Rijeka, Croatia: 2015.
-
- Olesen M.A., Quintanilla R.A. Chapter 20—Alcohol consumption induces oxidative damage, neuronal injury, and synaptic impairment: Consequences for the brain health. In: Martin C.R., Patel V.B., Preedy V.R., editors. Diet and Nutrition in Neurological Disorders. Academic Press; Cambridge, MA, USA: 2023. pp. 365–385.
-
- Pérez M.J., Loyola R., Canelo F., Aranguiz A., Tapia-Monsalves C., Osorio-Fuentealba C., Quintanilla R.A. NADPH oxidase contributes to oxidative damage and mitochondrial impairment induced by acute ethanol treatment in rat hippocampal neurons. Neuropharmacology. 2020;171:108100. doi: 10.1016/j.neuropharm.2020.108100. - DOI - PubMed
-
- Quintanilla R.A., Pérez M.J., Aranguiz A., Tapia-Monsalves C., Mendez G. Activation of the Melanocortin-4 Receptor Prevents Oxidative Damage and Mitochondrial Dysfunction in Cultured Hippocampal Neurons Exposed to Ethanol. Neurotox. Res. 2020;38:421–433. doi: 10.1007/s12640-020-00204-1. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

