Oral Administration of Propolis and Lysozyme Combination Improves Feline Oral Health and Modulates Systemic Inflammatory and Oxidative Responses
- PMID: 40563274
- PMCID: PMC12189585
- DOI: 10.3390/antiox14060639
Oral Administration of Propolis and Lysozyme Combination Improves Feline Oral Health and Modulates Systemic Inflammatory and Oxidative Responses
Abstract
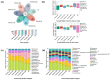
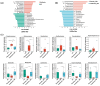
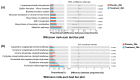
Oral diseases are highly prevalent among domestic cats, with microbiota dysbiosis as a primary etiological factor. However, effective microbiota-targeted interventions remain limited. This study evaluated the efficacy of a dietary supplement combining propolis and lysozyme (PL) in mitigating feline oral health issues, based on a cohort of 24 cats divided equally into placebo, treatment, and healthy control groups (n = 8 per group). Supragingival microbiota were analyzed via 16S rRNA gene sequencing, alongside assessments of volatile sulfur compounds (VSCs), oral health indices, and systemic inflammatory, oxidative, and immune markers. After 28 days of intervention, cats receiving PL supplementation demonstrated significant improvements, including a 35.4% reduction in VSCs and notable decreases in debris (34.9%), plaque (51.2%), and gingival indices (61.0%). Systemically, MDA and TNF-α levels decreased, while SOD, T-AOC, and IL-4 increased. Microbiota analysis revealed suppression of Porphyromonas and Selenomonas and enrichment of Moraxella and Bergeyella. Reductions in VSCs, gingival index, and TNF-α were correlated with lower Porphyromonas abundance, while Moraxella and Luteimonas were positively associated with antioxidant status. Functional predictions indicated downregulation of virulence-related pathways and increased expression of glutathione reductase. These findings highlight PL's potential as a natural, microbiota-based intervention that improves feline oral health and modulates the oral-systemic axis, supporting its application in integrative oral care strategies.
Keywords: feline; inflammatory; lysozyme; oral health; oral microbiota; oxidative responses; propolis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

