Acrylamide Neurotoxicity Studies in Caenorhabditis elegans Model
- PMID: 40563276
- PMCID: PMC12189583
- DOI: 10.3390/antiox14060641
Acrylamide Neurotoxicity Studies in Caenorhabditis elegans Model
Abstract
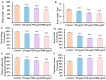
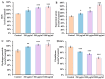
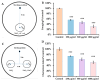
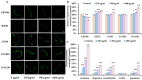
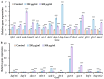
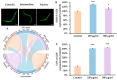
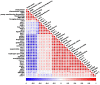
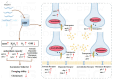
Acrylamide (ACR), utilized as a precursor for producing polyacrylamide for water purification, has demonstrated neurotoxic properties. However, the mechanisms underlying its neurotoxicity remain inadequately understood. In this investigation, Caenorhabditis elegans were exposed to ACR at concentrations ranging from 250 to 1000 μg/mL and then their locomotor behavior, neuronal development, neurotransmitter concentrations, and gene expression profiles were assessed. Exposure to 250-1000 μg/mL ACR resulted in observable behaviors such as head swiveling and body bending, accompanied by a significant reduction in body size. Furthermore, ACR exposure caused damage to serotonergic, cholinergic, dopaminergic, and glutamatergic neuronal structures. In this context, elevated levels of serotonin, dopamine, acetylcholine, and glutamate were detected, along with notable upregulation of the expression of genes associated with neurotransmitters, including tph-1, cat-4, mod-1, mod-5, cat-1, ser-1, dat-1, dop-1, dop-3, unc-17, cho-1, eat-4, and glr-2. Moreover, ACR exposure elevated reactive oxygen species (ROS), O2, and H2O2 levels while concurrently depleting glutathione (GSH), thereby compromising the antioxidant defense system. This led to a significant upsurge in the expression of genes involved in the nematode ACR detoxification pathway, specifically daf-16, skn-1, mlt-1, sod-3, gst-4, gcs-1, hsf-1, and hsp-16.2. Additionally, Spearman correlation analysis revealed a significant inverse relationship between certain neurotransmitter and antioxidant genes and locomotor activities, highlighting the role of these genes in mediating ACR-induced neurotoxicity in C. elegans. Collectively, this research enhances the understanding of the mechanisms related to ACR neurotoxicity.
Keywords: Caenorhabditis elegans; acrylamide; neurotoxicity; oxidative stress.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Exposure to benzotriazole UV stabilizer-329 at environmental concentrations induces neurotoxicity by affecting neurotransmission in Caenorhabditis elegans.Environ Pollut. 2025 Sep 15;381:126582. doi: 10.1016/j.envpol.2025.126582. Epub 2025 May 31. Environ Pollut. 2025. PMID: 40451527
-
Acute Cu exposure induces neurotoxicity via DAF-16/FoxO and SKN-1/Nrf2 pathway.J Environ Sci (China). 2025 Nov;157:489-500. doi: 10.1016/j.jes.2024.12.035. Epub 2024 Dec 28. J Environ Sci (China). 2025. PMID: 40602899
-
Hymenaea courbaril hydroalcoholic extract protects in vivo against oxidative stress in Caenorhabditis elegans via DAF-2 and SKN-1.J Toxicol Environ Health A. 2025 Jun 5:1-19. doi: 10.1080/15287394.2025.2514531. Online ahead of print. J Toxicol Environ Health A. 2025. PMID: 40471668
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.Health Technol Assess. 2008 Jun;12(28):iii-iv, ix-95. doi: 10.3310/hta12280. Health Technol Assess. 2008. PMID: 18547499
-
Etanercept and infliximab for the treatment of psoriatic arthritis: a systematic review and economic evaluation.Health Technol Assess. 2006 Sep;10(31):iii-iv, xiii-xvi, 1-239. doi: 10.3310/hta10310. Health Technol Assess. 2006. PMID: 16948890
References
-
- Hashem M.M., Abo-El-Sooud K., Abd El-Hakim Y.M., Badr Y.A., El-Metwally A.E., Bahy-El-Dien A. The impact of long-term oral exposure to low doses of acrylamide on the hematological indicators, immune functions, and splenic tissue architecture in rats. Int. Immunopharmacol. 2022;105:108568. doi: 10.1016/j.intimp.2022.108568. - DOI - PubMed
-
- Ji K., Kang S., Lee G., Lee S., Jo A., Kwak K., Kim D., Kho D., Lee S., Kim S. Urinary levels of N-acetyl-S-(2-carbamoylethyl)-cysteine (AAMA), an acrylamide metabolite, in Korean children and their association with food consumption. Sci. Total Environ. 2013;456:17–23. doi: 10.1016/j.scitotenv.2013.03.057. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

