The Nrf2 Activator CDDO-Imidazole Suppresses Inflammation-Induced Red Blood Cell Alloimmunization
- PMID: 40563311
- PMCID: PMC12189440
- DOI: 10.3390/antiox14060678
The Nrf2 Activator CDDO-Imidazole Suppresses Inflammation-Induced Red Blood Cell Alloimmunization
Abstract
Experimental Objective: During red blood cell (RBC) transfusion, inflammation promotes the production of anti-RBC alloantibodies that can cause significant hemolytic events. Avoiding RBC antigen exposure is the only strategy to prevent RBC alloimmunization in transfusion recipients. Identifying mechanisms that inhibit alloimmunization may lead to novel prophylactic interventions. One potential regulatory mechanism is the activation of the transcription factor nuclear factor erythroid-derived 2-like 2 (Nrf2), a master regulator of antioxidant pathways. Pharmacologic Nrf2 activators induce antioxidant production and improve the sequelae of inflammatory diseases. Thus, we tested the hypothesis that a Nrf2 activator, 1-[2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oyl]-imidazole (CDDO-Im), regulates inflammation-induced RBC alloimmunization.
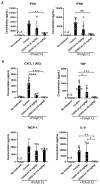
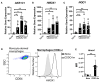
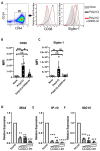
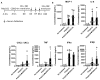
Methods: WT and Nrf2-deficient mice were treated with inflammatory stimuli and CDDO-Im prior to transfusion with RBCs expressing the KEL antigen (KEL+ RBCs). Anti-KEL IgM and IgG were measured in the serum of transfused mice. Nrf2-activated gene expression and interferon activity were measured in mice and human macrophages pre-treated with CDDO-Im and interferon stimuli.
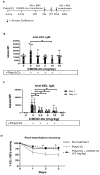
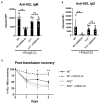
Results: Here, we report that CDDO-Im induces Nrf2-activated gene expression and inhibits type 1 interferon activity, which promotes RBC alloimmunization in transfusion models. In mice transfused with KEL+ RBCs, pre-treatment with CDDO-Im inhibited inflammation-induced anti-KEL antibody production and increased the post-transfusion recovery of KEL+ RBCs in a Nrf2-dependent manner. CDDO-Im also inhibited RBC alloimmunization in mice with pre-existing inflammation.
Conclusions: These results indicate that the activation of the Nrf2 antioxidant pathway regulates RBC alloimmunization to the KEL antigen in a pre-clinical model. If these findings translate to other models and human studies, Nrf2 activators may represent a potential prophylactic intervention to inhibit alloimmunization.
Keywords: Nrf2; RBC alloimmunization; inflammation; transfusion; type 1 interferons.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
CDDO-Imidazole regulates RBC alloimmunization to the KEL antigen by activating Nrf2.bioRxiv [Preprint]. 2025 Apr 6:2025.04.03.645598. doi: 10.1101/2025.04.03.645598. bioRxiv. 2025. PMID: 40235992 Free PMC article. Preprint.
-
IgG2b enhances alloantibodies to stored red blood cells.Transfusion. 2025 Aug;65(8):1408-1417. doi: 10.1111/trf.18306. Epub 2025 Jun 9. Transfusion. 2025. PMID: 40485608
-
HLA Class II regulation of immune response in sickle cell disease patients: Susceptibility to red blood cell alloimmunization (systematic review and meta-analysis).Vox Sang. 2022 Nov;117(11):1251-1261. doi: 10.1111/vox.13351. Epub 2022 Sep 14. Vox Sang. 2022. PMID: 36102140 Free PMC article.
-
Transfusion thresholds for guiding red blood cell transfusion.Cochrane Database Syst Rev. 2021 Dec 21;12(12):CD002042. doi: 10.1002/14651858.CD002042.pub5. Cochrane Database Syst Rev. 2021. PMID: 34932836 Free PMC article.
-
Type 1 Interferon Gene Signature Promotes RBC Alloimmunization in a Lupus Mouse Model.Front Immunol. 2020 Sep 25;11:584254. doi: 10.3389/fimmu.2020.584254. eCollection 2020. Front Immunol. 2020. PMID: 33101313 Free PMC article.
References
-
- (FDA) UDoHaHS Fatalities Reported to the FDA Following Blood Collection and Transfusion: Annual Summary for Fiscal Year. 2014. [(accessed on 11 November 2024)]. Available online: https://www.notifylibrary.org/sites/default/files/FDA%20Fatality%20Repor....
Grants and funding
LinkOut - more resources
Full Text Sources

