Dynamic Interplay Between Autophagy and Oxidative Stress in Stem Cells: Implications for Regenerative Medicine
- PMID: 40563323
- PMCID: PMC12189076
- DOI: 10.3390/antiox14060691
Dynamic Interplay Between Autophagy and Oxidative Stress in Stem Cells: Implications for Regenerative Medicine
Abstract
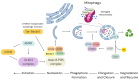
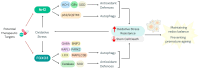
The crosstalk between autophagy and oxidative stress is a cornerstone of stem cell biology. These processes are tightly interwoven, forming a regulatory network that impacts stem cell survival, self-renewal, and differentiation. Autophagy, a cellular recycling mechanism, ensures the removal of damaged organelles and proteins, thereby maintaining cellular integrity and metabolic balance. Oxidative stress, driven by the accumulation of reactive oxygen species (ROS), can act as both a signalling molecule and a source of cellular damage, depending on its levels and context. The interplay between autophagy and oxidative stress shapes stem cell fate by either promoting survival under stress conditions or triggering senescence and apoptosis when dysregulated. Recent evidence underscores the bidirectional relationship between these processes, where autophagy mitigates oxidative damage by degrading ROS-generating organelles, and oxidative stress can induce autophagy as a protective response. This crosstalk is critical not only for preserving stem cell function but also for addressing age-related decline and enhancing regenerative potential. Understanding the molecular mechanisms that govern this interplay offers novel insights into stem cell biology and therapeutic strategies. This review delves into the intricate molecular dynamics of autophagy and oxidative stress in stem cells, emphasizing their synergistic roles in health, disease, and regenerative medicine applications.
Keywords: ROS; ageing; antioxidant; autophagy; mitophagy; regenerative medicine; stem cell.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

