CircRNA_1156 Attenuates Neodymium Nitrate-Induced Hepatocyte Ferroptosis by Inhibiting the ACSL4/PKCβII Signaling Pathway
- PMID: 40563332
- PMCID: PMC12189531
- DOI: 10.3390/antiox14060700
CircRNA_1156 Attenuates Neodymium Nitrate-Induced Hepatocyte Ferroptosis by Inhibiting the ACSL4/PKCβII Signaling Pathway
Abstract
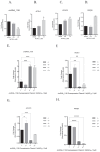
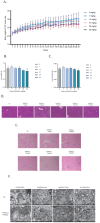
Ferroptosis, a form of regulated cell death driven by lipid peroxidation, has been implicated in the pathogenesis of liver diseases. This study investigates the role of circRNA_1156 in neodymium nitrate (Nd(NO3)3)-induced hepatocyte ferroptosis. Our in vitro experiments revealed that exposure to Nd(NO3)3 (1.2 µM) significantly reduced the viability of AML12 hepatocytes (p < 0.01), increased levels of reactive oxygen species (ROS) and malondialdehyde (MDA) (p < 0.001), and depleted glutathione (GSH) (p < 0.001). However, overexpression of circRNA_1156 effectively reversed these effects and suppressed the expression of ACSL4 and PKCβII (p < 0.01). In our in vivo experiments, chronic exposure to Nd(NO3)3 (7-55 mg/kg for 180 days) induced hepatic iron deposition, mitochondrial damage, and activation of the ACSL4/PKCβII pathway (p < 0.01). These adverse effects were significantly ameliorated by circRNA_1156 overexpression (p < 0.05). Our findings identify circRNA_1156 as a novel inhibitor of Nd(NO3)3-induced ferroptosis via downregulation of the ACSL4/PKCβII pathway, providing valuable therapeutic insights for hepatotoxicity caused by rare earth element compounds.
Keywords: ACSL4/PKCβII pathway; circRNA_1156; ferroptosis; hepatocyte injury; neodymium nitrate.
Conflict of interest statement
The authors declare that this study was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures





References
-
- Hu X.F., Jiang J. Ferroptosis and cancer: Interlinkages and potential applications. Clin. Transl. Rep. 2023;1:12–20. doi: 10.58832/ctr.2023.7.6.2. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

